Introduction
Acute pancreatitis (AP) is an inflammatory process resulting from various aetiologies that initiate as a local acinar cell injury and may subsequently involve various other organ systems, leading to organ failures. It is usually a self-limiting disease, however, approximately one-fourth of cases may present as or progress into severe acute pancreatitis (SAP), which is associated with increased mortality.1 Thus, predicting the nature of disease on admission is crucial for guiding management and improving outcome. In clinical practice, several scoring systems are available, including the Ranson score, Glasgow score, Acute Physiology and Chronic Health Evaluation (APACHE II), Sequential Organ Failure Assessment (SOFA) score, Bedside Index for Severity in Acute Pancreatitis (BISAP) score, and modified Computed Tomography Severity Index (CTSI)2-4. However, these systems require numerous parameters, making it cumbersome to calculate them all in the early stages of the disease5. Other simple tests, including serum markers like procalcitonin, interleukin6, and interleukin-8, have been studied previously, however these are expensive, lack validation, and may not be readily available6,7.
White blood cell count is a common haematological test included in many risk assessment scores for acute pancreatitis and other medical and surgical emergencies. Neutrophils play a role in propagating the systemic inflammatory response syndrome (SIRS) and the inflammatory cascade in AP, while lymphocyte depletion is observed in severe sepsis and is associated with a poor outcome8-10. Neutrophils and lymphocytes better reflect the immune response than the total WBC count, and the neutrophil-to-lymphocyte ratio (NLR) has recently emerged as a marker of systemic inflammation. Compared to other inflammation markers, NLR is simpler and less costly to measure, making it a potential independent predictor of the severity of pancreatitis. However, the literature regarding the prognostic value of NLR in acute pancreatitis is not very clear.
The aim of this retrospective study was to analyze the value of NLR in predicting the severity of acute pancreatitis and to compare it with BISAP score and modified CT-severity index.
Methods
We conducted a retrospective study at our tertiary care centre. The electronic medical record (EMR) facilitates the retrieval of clinical and laboratory data of all patients. The study was undertaken with the approval of the local research ethics committee and in accordance with the Declaration of Helsinki (1989) of the World Medical Association. The requirement for informed consent was waived in view of the retrospective nature of this study.
Study Population
Patients aged >18 years admitted with a diagnosis of acute pancreatitis from January 2015 to December 2019 were included. Subjects with underlying chronic pancreatitis were excluded from the study.
Data Collection
Data was retrieved from the well-equipped EMR system of the institute. Demographic data, including gender and age was captured. Clinical details regarding the aetiology of pancreatitis, co-morbidities (diabetes mellitus and hypertension), duration of ICU stay, and total hospital stay were recorded. Haematological, biochemical and imaging (X-ray chest and CT-abdomen) details were collected. All laboratory investigations were analysed within 30 min after sample collection.
Definitions
As per the revised Atlanta criteria, the diagnosis of AP requires 2 of the following 3 criteria11: typical abdominal pain; serum amylase or lipase elevation = 3 times the upper limit of normal; and characteristic findings of AP on imaging (contrast-enhanced CT, MRI or abdominal ultrasonography). Mild acute pancreatitis, the most common form, has no organ failure, local or systemic complications. Moderately severe acute pancreatitis is defined by the presence of transient organ failure, local complications, or exacerbation of co-morbid disease3. Severe AP is defined by persistent organ failure, i.e., organ failure >48 hours.
White blood cell differential at the time of admission was analyzed and NLR was determined by the ratio of absolute neutrophil and lymphocyte count. BISAP score and modified CTSI were derived from collected clinical and imaging data. The cut-off value of NLR in the study population was derived from the ROC curve, whereas validated indices were used for the BISAP score (>2) and modified CTSI [mild (0-3 points); moderate (4-6 points); severe 7-10 points)] for analysis11,12.
Statistical Analysis
Data analysis was performed using SPSS version 22.0. Quantitative variables were expressed as the mean and standard deviation, while qualitative variables were summarized as frequency and percentages. Comparison of continuous variable between two group were analyzed by student t-test, and comparison of quantitative variables among more than two groups were analyzed by ANOVA. Association between qualitative variables were analyzed by Chi-square test, and relationship between quantitative variables was analyzed by Pearson correlation. An ROC curve was plotted to assess the optimum cut-off value of the predictive variable to categorize the outcome variable. The area under the curve with standard error and 95% CI of AUC was calculated. Sensitivity, Specificity, PPV NPV and accuracy of the variable were calculated for each cut-off value. Ap-value of <0.05 was considered as statistically significant.
Results
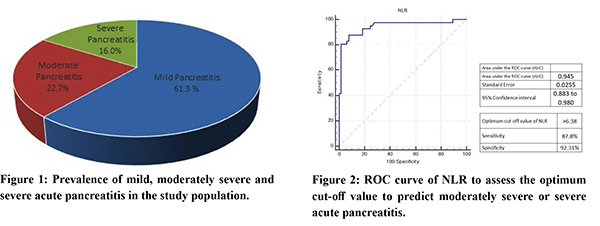
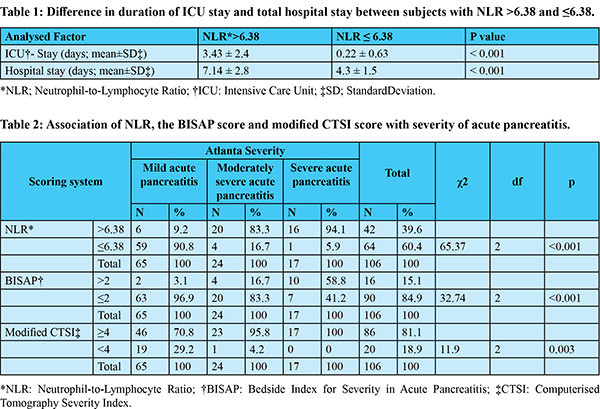
Among 106 subjects with acute pancreatitis 71.6% (n=77) were men. The most common aetiology for pancreatitis was alcohol, followed by gallstone disease (supplementary Table 1). The mean time of presentation after symptom onset was 5 days (SD ±2 days). Mild pancreatitis was seen in 61.3% (n=65) of subjects, moderately severe pancreatitis in 22.7% (n=24) and 16% (n=17) patients had SAP (Figure 1). The optimum cut-off value of NLR >6.38, derived from the ROC curve, was associated with a sensitivity of 87.8% and specificity of 92.3% (AUC 0.945; 95% CI 0.88 to 0.98) in predicting the development of moderate or severe AP (Figure 2). In patients with NLR >6.38, the duration of ICU stay and total hospital stay also was significantly high (Table 1). In our study population, the sensitivity and specificity of the BISAP score (>2) in the prediction of moderately severe and severe AP were 78% and 92.3% (AUC 0.881; 95% CI 0.80 to 0.94), respectively, whereas modified CTSI (=4) score could predict with 80.5% sensitivity and 87.7% specificity (AUC 0.89; 95% CI 0.82 to 0.94) (Figure 3). Both the BISAP score and modified CTSI score were significantly correlating with NLR in our study (supplementary Table 2 and 3), and all three scores were associated with the severity of pancreatitis (Table 2). Binary logistic regression analysis revealed that higher NLR (>6.38) was independently associated with the development of severe acute pancreatitis (Table 3). There was no significant difference in the distribution of age, sex, aetiology of pancreatitis and the presence of comorbidities (diabetes mellitus and hypertension) between mild and severe acute pancreatitis groups (supplementary Table 4). There was no mortality during the admission period among our study population.

Discussion
In the present study, we found that a cut-off value of NLR >6.38 on admission could predict the development of moderately severe or severe acute pancreatitis with a sensitivity and specificity of 87.8% and 92.3%, respectively. This is superior to both the BISAP and modified CTSI scores. Although the total WBC count is a compositional element of Ranson’s criteria, Glasgow score, APACHE-II, and BISAP scores, the total WBC count itself, unlike CRP or blood urea nitrogen, is not assessed as an independent marker for predicting the prognosisof acute pancreatitis2-4. However, NLR has been identified by several previous studies as an index that reflects the prognosis of various benign inflammatory or malignant diseases,13 including acute pancreatitis.
In the literature, various studies have highlighted the importance of NLR in the prognostication of acute pancreatitis; however, there is significant diversity in determining the cut-off value. For instance, a Korean study reported different NLR cut-off values for gallstone pancreatitis and alcoholic pancreatitis (32.4 ± 30.9 vs. 9.8 ± 8.8) in severity assessment.14 In contrast, a UK-based study derived optimal cut-off from ROC curves as 10.6 (day 0), 8.1 (day 1), and 4.8 (day 2), yielding sensitivities of 63-90%, specificities of 50-57% for risk prediction in pancreatitis.15 Our analysis showed that an NLR on day0 of the hospital admission (>6.38) was significantly associated with the manifestation of SAP. Azab et al. suggested a relationship between NLR and the duration of intensive care16. In our observation, the number of days spent in intensive care and the total duration of hospital stay were also significantly higher in subjects with high NLR.
Initially, the importance of NLR in inflammatory conditions was described by Zahorec et al10. An increase in neutrophil numbers corresponds with the development of SIRS and organ failures, which are hallmarks of SAP. Lymphocyte numbers usually increase following the initial stress and mediate the subsequent inflammatory response. The traditional view is that neutrophilia is the primary cause of an elevated NLR, SIRS, and poor prognosis, while lymphocyte count remains static. However, Suppiah et al15 studied that lymphopenia within 24 hours of admission and persistent lymphopenia beyond this period is just as much a contributor to increased NLR and poor prognosis as neutrophilia. This is replicated in other studies where persistent lymphopenia is an independent marker of progressive inflammation.
The BISAP score was proposed in 2008 for the early recognition of patients with SAP at risk for mortality. This 5-point scoring system comprises of five variables: blood urea nitrogen level >25 mg/dl, impaired mental status, the development of SIRS, age >65 years and the presence of pleural effusion. It is a simple and accurate method for the early identification of acute pancreatitis at increased risk of morbidity and mortality5. The derivation of NLR is much simpler. Liu G et al.5 have proven that NLR is not inferior to the BISAP score in pancreatitis risk stratification. Our study also showed that NLR is an independent prognostic marker for acute pancreatitis on binary logistic analysis.
Contrast enhanced CT (CECT) is an excellent imaging modality for diagnosis and establishing the extent of the disease process and grading its severity. Modified CTSI has been proven as a reliable indicator for the assessment of pancreatitis severity.12 However, the usual recommended timing for imaging analysis is 48 hours after admission, making it less useful for early assessment of the disease. In our study, we found that modified CT severity index score was significantly correlating with NLR value, and both scores were significantly associating with the development of SAP.
Our study has certain limitations, such as being single-center and retrospective. Only day-0 of hospital admission values were considered for the assessment of NLR and the BISAP score. The relationship of disease severity with the dynamicity of these scores could not be evaluated. Abdominal imaging (CT scan) was done after admission at different time interval, leading to a lack of uniformity in the values of modified CTSI score in our study population.
Conclusion
NLR is a simple, inexpensive, and easy-to-carry out prognostic tool for predicting the severity of pancreatitis, which is not inferior to either the BISAP score or modified CTSI score in this retrospective study. However, large-scale multicentre studies are needed to derive and to validate an accurate cut-off value of NLR for the risk prediction in patients of acute pancreatitis.
References
- Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol 2010;105: 435–41.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. Am J Gastroenterol1974; 61: 443–51.
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62: 102–11.
- Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute ancreatitis. Lancet 1989;2:201–5.
- Liu G, Tao J, Zhu Z, Wang W. The early prognostic value of inflammatory markers in patients with acute pancreatitis. Clin Res Hepatol Gastroenterol. 2019;43(3):330-7.
- Aoun E, Chen J, Reighard D, Gleeson FC, WhitcombDC, Papachristou GI. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology 2009;9(6): 777–85.
- Mofidi R, Suttie SA, Patil PV, Ogston S, Parks RW. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery 2009;146(1): 72–81.
- de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care 2010;14(5):R192.
- Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 2002;18(6): 487–494.
- Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102(1): 5–14.
- Singh V, Wu BU, Maurer R, Repas K, Maurer R, Johannes RS, et al. A prospective evaluation of the Bedside Index of Severity in Acute Pancreatitis. Am J Gastroenterol 2009; 104:966-71.
- Banday IA, Gattoo I, Khan AM, Javeed J, Gupta G, Latief M. Modified Computed Tomography Severity Index for evaluation of acute pancreatitis and its correlation with clinical outcome: A tertiary care hospital based observational study. J Clin Diagn Res. 2015;9(8):TC01-TC5.
- Varim C, Acar BA, Uyanik MS, Acar T, Alagoz N, Nalbant A, et al. Association between the neutrophil-to-lymphocyte ratio, a new marker of systemic inflammation, and restless legs syndrome. Singapore Med J. 2016;57(9):514-516.
- Cho SK, Jung S, Lee KJ, Kim JW. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterol. 2018;18(1):18.
- Suppiah A, Malde D, Arab T, Hamed M, Allgar V, Smith AM, et al. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. 2013;17(4):675-681.
- Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, et al. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-452
- Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study. Medicine (Baltimore). 2019;98(16):e15275.