Introduction
Gastrointestinal stromal tumors (GIST) are rare tumors with a potential for cure in the vast majority1. Gastroesophageal junction GISTs (GEJG) are currently classified under gastric GISTs. R0 resection offers the only chance of cure along with tyrosine kinase inhibitors (TKI) where indicated2.The management guidelines for GEJG are currently an extrapolation of those for gastric GISTs.
In patients with a locally advanced disease precluding a resection with minimal morbidity, TKI are used with an intent to downstage the disease before surgery. In patients with advanced disease (peritoneal, liver metastasis etc.) precluding surgical resection for cure, current National Comprehensive Cancer Network (NCCN)guidelines recommend long term TKI with palliative resection for tumor related complications3.
Majority of gastric GISTs are resectable laparoscopically but GEJG pose certain unique challenges owing to their location. In the present study, pre-operative diagnostic aspects, details of surgical techniques employed, need for adjunct procedures (anti-reflux, pyloroplasty), and strategy for use of TKI in patients undergoing surgery for GEJG were evaluated. The purpose of the study was to analyse the factors affecting recurrence and the effect of high risk of progression (HROP) on management strategy in in these patients.
Methods
Patients undergoing surgical resection for GEJG during the period June 2007 to September 2020, by the senior author (GS) formed part of the study group. Data of these patients were retrospectively reviewed from a prospectively maintained database. An informed consent was obtained from all patients. The retrospective nature of study and analysis of available data did not necessitate an institutional review board approval. Demographic details, clinical presentation, investigations performed, surgical details, use of TKI in the neoadjuvant and adjuvant setting were studied. Operative details included the laparoscopic surgical techniques employed, details of additional procedures (anti-reflux, pyloroplasty, liver metastatectomy etc.), duration of surgery, blood loss, postoperative morbidity and mortality. Histopathology of resected specimens were reported as per College of American Pathologists (CAP) guidelines4. Adjuvant therapy with TKI was given in those with HROP as per NCCN guidelines. Patients were followed up with clinical evaluation, routine labs and an ultrasound abdomen six monthly along with a MDCT abdomen annually till five years and only clinical evaluation and annual ultrasound, thereafter. Follow-up data was collected from follow-up outpatient records and the last follow-up was recorded by telephonic contact on 1st August 2021.
Statistical Analysis
This was done with SPSS version 22 (IBM SPSS Statistics, Somers NY, USA). Categorical data was represented in the form of frequencies and proportions. Chi-square test or Fischer’s exact test (for 2x2 tables) was used as test of significance for qualitative data. Continuous data was represented as mean and standard deviation. Independent T test and Mann Whitney U test was used as test of significance to identify the mean difference between two quantitative variables and qualitative variables respectively. ‘p’ value(Probability that the result is true) of <0.05 was considered as statistically significant after assuming all the rules of statistical tests.
Results
Forty four patients diagnosed to have gastrointestinal GISTs were operated during the study period of whom there were 28 gastric, 5 duodenal, 8 Jejunal, 2 Ileal and 1 colonic. Of the 28 gastric GIST, 14 were classified as GEJG and formed part of the study group. The demographic features, clinical presentation, upper gastrointestinal endoscopy (UGIE), MDCT findings, location and morphological classification of the tumors are shown in Table 1. Endoscopic ultrasound (EUS) was done in five patients along with biopsy in 4 (28%). Preoperative biopsy (four and two patients on EUS and UGIE) was available in six patients, with spindle cells in all of them on histology. Immunohistochemistry (IHC) confirmed GIST in 3(CD 117 positive) and tissue was insufficient in remaining three patients.
Surgical Procedures
Thirteen patients had upfront surgery and one patient had neo-adjuvant imatinib mesylate (IM) prior to surgical resection. All patients underwent laparoscopic resections successfully. The types of resections were stapled exogastric resection, gastrotomy and endogastric staple resection followed by closure of gastrotomy by sutures/staples, lesser curve sleeve resections, gastrotomy-eversion and stapled resection, proximal gastrectomy akin to standardized techniques described by Kong and Yang5. Case 5, had a bulky mass arising from the posterior wall adjacent to GEJ with infiltration into the spleen that was managed with an enbloc proximal gastrectomy and upper pole splenectomy and a pyloroplasty. UGIE during surgery was done in all patients; to localize tumor during surgery in two and to check integrity of staple/suture line after resection in the remaining (including one patient who underwent a proximal gastrectomy). An esophageal bougie was placed prior to resection in 12 (86%) of patients. The use of the bougie during resection avoided an inadvertent narrowing of the GEJ while resecting tumor (exogastric/endogastric) or closure of gastrotomy. In two of the patients, bougie was not placed; in one requiring proximal gastrectomy and the other where an exogastric resection was done for a fundal tumor in whom the staple line was one cm away from GEJ.
Additional Surgical Procedures
Five patients required a hiatal repair. Fundoplication was performed in four of these patients (Toupet in 3 and Dorr in one). Heineke-Mikulicz pyloroplasty was performed in two patients; one patient who underwent a proximal gastrectomy and the other who had a lesser curve sleeve resection. One patient each underwent a segment four liver lesion metastatectomy and a cholecystectomy.
Hospital Course
The mean operative time was 126 (+/- 38) minutes and blood loss was 20 (+/- 12) mL. Oral intake was resumed on the evening of surgery in 5, postoperative day 1 in 5 and postoperative day 2 in 4. There was no postoperative morbidity or mortality.
Histopathology, Immunohistochemistry and Risk of Progression
Histopathology showed an R0 resection in all. The CAP protocol findings including procedure, tumor size, focality, mitotic rate, histologic grade, risk assessment, TNM staging are shown in Table-2. Immunohistochemistry revealed positivity of CD117 in 86% (12/14), CD34 in 71% (10/14), DOG1 in 83% (10/12), SMA in 14% (2/14), Desmin in 14% (2/14) and S100 in none.
The risk assessment showed very low risk in eight, low risk in one, intermediate risk in three and high-risk of progression (HROP) in two patients. Of two patients with HROP on final histopathology (Case1 and Case13), although preoperative biopsies were done in both, the ‘HROP status’ was established in second patient,while in the first patient it was inconclusive. In the former patient, the biopsy was done at upper gastroscopy while in the latter had an endo-sonography guided trucut biopsy. Thus, ‘HROP status’ was evident in the first patient on final histopathology alone.
Neo-adjuvant and Adjuvant Therapy
One patient (case 13) received neoadjuvant IM therapy. Six patients received adjuvant IM (case 1, Case 4, Case 5, Case 10, Case 11, and Case 13). IM was discontinued after three months in Case10 because of intolerance to the drug.
Follow-up
All patients were available for follow-up. Twelve patients remain disease free at median follow-up of 91 (8-161) months. Case 5 had significant post-operative reflux symptoms and has been on longterm prokinetics. Two patients developed recurrence (Case 1 and Case 13). The first patient had a sleeve resection (gastrotomy, eversion and wedge resection). He discontinued IM after one year and presented with omental recurrence 6 months later. IM was restarted and 3 months later he underwent laparoscopic resection of the omental lesion, with further continuation of IM for 3 years. Histopathology revealed myxoid degeneration with no viable cells in the omental lesion. At 161 months from the index surgery and 110 months after discontinuing IM, he remains asymptomatic with no evidence of recurrence on imaging. In the second patient (tumor size of 8cms), the HROP status was available on preoperative endoscopic biopsy. His imaging had shown locally advanced disease (GEJG with extension along lesser curve and infiltration into right crus of diaphragm, body of pancreas and with solitary liver metastasis). He was started on IM and a multidisciplinary team (MDT) review at three months follow-up recommended a total gastrectomy, distal pancreatectomy with splenectomy and liver metastasectomy, which was refused by the patient. He was continued on IM and follow up scans at 18 months revealed significant regression of primary tumor (no adjacent organ infiltration) with regression of the liver nodule and was now recommended a minimally invasive resection by the MDT. He underwent a synchronous lesser curve gastric sleeve resection of the tumor with segment IV liver metastasectomy, Toupetfundoplication with pyloroplasty. Histopathology showed myxoid degeneration with no viable tumor. IM was continued for further one and half years (a total of three years). After a three-year asymptomatic period from surgery, he presented with epigastric pain and PET-CT imaging at thirty-four months post-surgery (sixty-two months from diagnosis) showed a local recurrence and solitary liver metastasis (segment III). A EUS guided biopsy confirmed a recurrence with similar histopathology and IHC as the index biopsy. There were no actionable mutations on molecular analysis. He was restarted on IM and remains under followup.
Factors Determining Risk of Recurrence
On comparing the various factors between patients developing recurrence and those that did not, the factors which significantly increased chance of recurrence were lesions more than 5 cm, ulcerated lesions and those which showed a HROP (Table 3).
Discussion
GEJG are currently grouped under gastric GISTs, and their management strategy is an extrapolation of the current NCCN guidelines for gastric GISTs in general3. The goal of surgery in patients with GEJG is an organ preserving sleeve gastric R0 resection. The feasibility of laparoscopic resection of GEJG has been demonstrated by several studies6,7. Unlike Gastric GIST, resection of GEJG, pose certain risks, viz; the potential for narrowing the GEJ, disruption of the hiatus and lower esophageal sphincter complex, vagal nerve injury and greater risk of leak when suture/staple line extends on to the esophagus, to name a few. There is paucity of data providing specific recommendations for patients with GEJG. Majority of patients with GISTs experience a long-term survival and it would be important to address the above issues, to give a better long-term quality of life in these patients. Based on current recommendations, to avoid major morbidity, patients with resectable GEJG tumors requiring total gastrectomy are generally considered for neo-adjuvant therapy to downstage the disease and enable a proximal gastric resection3. However, patients undergoing a proximal gastrectomy too would be expected to have a greater morbidity when compared to a sleeve gastric resection and downstaging the disease with neo-adjuvant therapy in such patients to enable a sleeve resection has not been studied. Additionally,identifying ‘HROP status’ among patients in whom sleeve gastric resection is feasible and thereby offering neo-adjuvant treatment to such patients has not been addressed clearly.
Surgical Considerations
In small lesions close to the GEJ, a stapled sleeve resection is often possible with no exposure of tumor to general peritoneal cavity. In tumors where such a sleeve resection tends to encroach on to GEJ, a gastrotomy adjacent to tumor with eversion of tumor through the gastrotomy might enable a stapled sleeve resection. Also, excising the tumor with a margin and closing the gastrotomy with stapler/suture is another well described technique6,7. In our study, a sleeve gastric resection was possible in 93% (13/14) patients. Tumor rupture is associated with high incidence of recurrence. Patients with an ulcerated or umbilicated lesion on the mucosal surface are not considered as tumor rupture. However, exposing such tumors by eversion through a gastrotomy and thereby to the general peritoneal cavity are categorized under ‘tumor rupture8. An unprotected tumor, ulcerated on the mucosal side can therefore increase the chance of local recurrence or dissemination. Both patients in our study who developed recurrence had ulcerated tumors and required a gastrotomy during resection which would categorize them as ‘perforated tumors’, which could explain recurrence in them. It is debatable whether this risk in such patients, is more with laparoscopic surgery when compared to open surgery.
While a sleeve gastric resection that preserves the GEJ is appropriate and feasible in most patients with GEJG, proximal gastrectomy should be reserved for a few patients where preserving GEJ is not possible. Although a proximal gastrectomy is a partial gastric resection, its long-term morbidity of reflux and esophagogastric anastomotic stricture would make this procedure an overkill for most patients with GEJG. Data from patients with adenocarcinoma of proximal stomach has shown the preference of total gastrectomy over proximal gastrectomy because of long term complication of reflux and anastomotic stricture seen with the latter procedure9. A longer survival is seen in patients with GISTs in general but there is limited data to address the issue of whether, neoadjuvant treatment (TKI) would downstage the disease to enable a GEJ preserving sleeve (wedge) resection and avoid a proximal gastrectomy in this group of patients.
Some of the patients with tumors near GEJ would require mobilization of lower esophagus and GEJ to facilitate a sleeve gastric resection. This was required in 5 patients in our study. There is likelihood of disruption of the physiological anti-reflux mechanism and therefore a hiatal repair/ anti-reflux procedure would be appropriate in them. Blum et al, have reported resection of lower esophageal GIST tumors where resection crossed GEJ with fundoplication in two of their patients10. Again, there is inadequate data with regards to the incidence of reflux and recommendations to address this during surgery for GEJG. A hiatal repair with a Dorr or Toupet fundoplication seem appropriate in patients with suture/staple lines across the anterior or posterior GEJ respectively. Lymph nodal dissection during resection for GIST is seldom required and the plane of dissection remains close to the stomach wall. Therefore, tumors in the vicinity of lesser curve should have minimal disruption of the vagal innervation. However, when an extensive dissection is carried out in patients with large tumors or those requiring a proximal gastrectomy, complete vagal denervation inevitably occurs. These patients merit consideration for a drainage procedure, namely a pyloroplasty and this was done in two of our patients.
Approach in Patients with T4 Disease Detected at Surgery
In patients with T4 disease, laparoscopic enbloc resection for GEJG is feasible. Current guidelines recommend use of IM in locally advanced tumors detected on imaging. In one of our patients (case no 5), an upper pole splenic infiltration was detected during surgery, and we proceeded to do an enbloc proximal gastrectomy with upper pole splenectomy.The large size and the disposition of the tumor with upper pole splenic infiltration precluded a sleeve gastric resection. An enbloc resection seemed reasonable in this patient, since an R0 resection could be achieved by preserving the spleen. Interestingly, at final histology the splenic infiltration was found to be inflammatory. In patients deemed resectable who undergo surgery and the ‘T4 status’ becomes evident during dissection, it is unclear whether one should proceed with an enbloc resection or abandon the procedure, with a plan for IM therapy followed by reassessment for resection.
Preoperative Risk Stratification
In the present study, high risk GISTs (n=2) were characterized by size greater than 5 cms, ulceration of mucosa, necrosis on histology, mitosis more than 5/5mm2, recurrence at 13-36 months.The ROP assessment is the most important predictor of local recurrence, metastasis and tumor related death, that is in most cases made on histopathology following surgical resection in the vast majority of resectable cases 11. Currently, a preoperative trucut biopsy is not recommended in those patients with lesions that more than 5 cm that are resectable with minimal morbidity3,12. Our study found that two of the three patients with tumors larger than 5 cm developed recurrence and both these patients had HROP based on CAP protocol4. Based on imaging findings, case 1 went for upfront surgery while case 13 who had locally advanced disease with solitary liver metastasis on imaging, received neo-adjuvant IM. It is notable that the treatment plan was based on ‘imaging assessment’ of locally advanced nature and not based on the detection of ‘HROP status’ that was incidental on preoperative biopsy in case 13. Thus, with an intention to treat for cure, patients with locally advanced disease and those with resectable metastasis are selected for neo-adjuvant IM, while others with tumors resectable with minimal morbidity are offered upfront surgery. To emphasise, currently, ROP status is not a factor taken into consideration while deciding neoadjuvant treatment despite recognizing it as an important determinant for recurrence3. It is evident thata preoperative biopsy in patients with a tumor size greater than 5cm, could identify a subset of patients as HROP. None of the tumors less than 5 cm would qualify by definition for ‘HROP status’. Knowing the HROP status helps in three ways; firstly, to select out patients for neoadjuvant IM; secondly, in surgical decision making and thirdly in prognostication. Further, we would recommend against the techniques that employ gastrotomy in resecting GEJG that have ulceration and a ‘HROP’. Also, we suggest a low threshold for conversion to an open operation to complete the operation when a gastrotomy is required in such patients. Currently, there is no prospective study to conclude the equivalence of long-term oncological outcomes between laparoscopic and open surgery groups for those patients with HROP and such a study would be possible only when the ‘HROP’ status is established preoperatively.
The goal of surgical resection is to achieve a ‘R0” status but even a ‘R1’ status has been shown to have no influence on recurrence free survival (RFS) in several studies on multivariate analysis13. Current guidelines, therefore recommend adjuvant therapy with IM and not re-resection (to achieve a R0) in patients with ‘R1’ status. It follows that,‘R0/R1’ status is of lesser importance than ‘HROP’ status in determining long-term outcome (recurrence) in these patients. The current recommendations for preoperative biopsy (EUS guided) are for lesions less than 2 cms, with the intent of observation in very low/low risk groups and surgery in others4. In summary, a preoperative biopsy in patients with tumor size more than 5cms will be needed to identify those with HROP and offer neoadjuvant therapy for them (Figure 1).
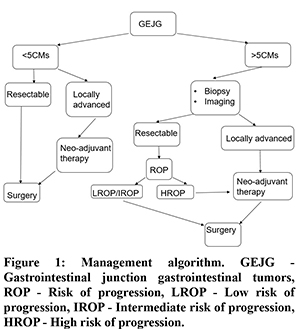
The current study has a relatively small cohort of patients with GEJG managed over a ten year period. Our study group represents only patients referred to us for surgical resection and the inherent selection bias excludes those patients that would remain under observation only or those with unresectable disease. However, the issues discussed herein are relevant for those patients considered for surgical resection of GEJG. There is an obvious need for larger prospective studies that could provide management guidelines that are specific for GEJG.
Conclusion
GEJG accounted for over 50% of gastric GISTs. Laparoscopic sleeve resection was feasible in majority of patients. Patients requiring a hiatal dissection to facilitate resection should have a hiatal repair and a partial fundoplication and those requiring extensive mobilization of lesser curve to enable resection merit consideration for a pyloroplasty. A preoperative biopsy in patients with lesions >5 cm in size would identify patients with HROP. Patients with HROP should be treated with neo-adjuvant therapy. A gastrotomy must be avoided in patients with HROP status undergoing surgical resection. HROP status appears to be of greater importance than R0 status in determining long-term outcomes in these patients. A large prospective multi-centre study would be able to address the need for sub-classification of these tumors from gastric GISTs and provide management guidelines.
Acknowledgements
Authors would like to acknowledge, Dr Mahesh V – Biostatistician, Associate Professor, Department of Community Medicine, CIMS, Chamarajnagar, Karnataka, India for his help in statistical analysis and Prof Ananthakrishnan N, Professor of Surgery, Mahatma Gandhi Medical College, Puducherry, India for reviewing the manuscript and his inputs.
References
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004 Sep 15;22(18):3813-3825.
- Garlipp B, Bruns C, J: State of the Art in the Treatment of Gastrointestinal Stromal Tumors. Gastrointest Tumors 2014; 1:221-236.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastrointestinal Stromal Tumors (GISTs). NCCN.org. Available at https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf. Version 1.2021 — October 30, 2020; Accessed: March 16, 2021
- College of American pathologists. CAP protocol for the examination of resection specimens from patients with Gastrointestinal stromal tumor. Version: GIST resection 4.1.0.0-August 2019; Accessed:May 2020.
- Kong SH, Yang HK. Surgical treatment of gastric gastrointestinal stromal tumor. J Gastric Cancer. 2013 Mar;13(1):3-18.
- Ma JJ, Hu WG, Zang L, Yan XW, Lu AG, Wang ML, Li JW, Feng B, Zhong J, Zheng MH. Laparoscopic gastric resection approaches for gastrointestinal stromal tumors of stomach. Surg LaparoscEndoscPercutan Tech. 2011 Apr;21(2):101-105.
- Xiong, W., Zhu, J., Zheng, Y. et al. Laparoscopic resection for gastrointestinal stromal tumors in esophagogastric junction (EGJ): how to protect the EGJ. Surg Endosc. 2018;32, 983–989.
- Nishida, T., Hølmebakk, T., Raut, C.P. et al. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2019; 26, 1669–1675.
- Rosa, F., Quero, G., Fiorillo, C. et al. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer–GIRCG). Gastric Cancer. 2018;21, 845–852.
- Blum MG, Bilimoria KY, Wayne JD, de Hoyos AL, Talamonti MS, Adley B. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg. 2007 Nov;84(5):1717-1723.
- Miettinen, Markku MD; Sobin, Leslie H MD†; Lasota, Jerzy MD Gastrointestinal Stromal Tumors of the Stomach, The American Journal of Surgical Pathology: January 2005;29(1):52-68.
- Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up.[Online, 21 September 2021].ESMO Guidelines Committee, EURACAN and GENTURIS.
- Rutkowski P, Skoczylas J, Wisniewski P: Is the Surgical Margin in Gastrointestinal Stromal Tumors Different. Visc Med 2018; 34:347-352.