|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
Hepatitis C virus, chronic renal failure, end stage renal disease, chronic kidney disease, dialysis, interferon |
|
|
Ajay Duseja,1 Narendra S Choudhary,1 Sachin Gupta,1 Radha K Dhiman,1 Yogesh Chawla,1 Vinay Sakhuja2
Departments of Hepatology1 and
Nephrology,2
Postgraduate Institute of Medical
Education and Research,
Chandigarh - 160012, India.
Corresponding Author:
Dr. Ajay Duseja
Email: ajayduseja@yahoo.co.in
DOI:
http://dx.doi.org/10.7869/tg.2012.47
Abstract
Introduction: Treatment of patients with chronic hepatitis C (CHC) is difficult in the setting of end stage renal disease (ESRD). The present study aimed to analyze the treatment outcome in patients with CHC and ESRD, being evaluated for kidney transplantation.
Methods: Data of 65 patients of ESRD with CHC (males: 53, mean age: 39.2±14.4 years) was analysed retrospectively. Patients were treated with either pegylated or conventional interferon (IFN) without ribavirin. Treatment response was assessed for rapid virological response (RVR), early virological response (EVR), end of treatment response (ETR) and sustained virological response (SVR).
Results: All patients were receiving hemodialysis (duration 1-60 months). Sixteen patients (25%) (genotype 1: 11, genotype 3: 4, genotype 2: 1) agreed for treatment (13 pegylated IFN and 3 conventional IFN). RVR was achieved in 7 patients (44%) and out of 11 patients (69%) who achieved EVR, ETR was achieved in 7 (44%) patients. Seven patients (44%) dropped out during treatment (2 because of side effects). SVR could be demonstrated in one of 7 patients who achieved ETR (6 patients were lost to
follow up after ETR).
Conclusions: In our experience, dropouts before, during and after treatment are a major problem in patients with CHC and ESRD. Of those who complete treatment, around half of them are able to achieve the end of treatment response.
|
48uep6bbphidvals|532 48uep6bbph|2000F98CTab_Articles|Fulltext The prevalence of chronic hepatitis C (CHC) is higher in patients with end stage renal disease (ESRD) in comparison to the general population, and is probably related to inadequate infection control in dialysis units. In India, the prevalence of CHC in patients with chronic kidney disease (CKD) is reported to be around 12-40% across different centers.[1] CHC not only can cause hepatitis C virus (HCV) associated glomerulonephritis (GN) and cryoglobulinema, but is also responsible for increased morbidity and mortality due to cirrhosis and hepatocellular carcinoma in patients with ESRD who undergo renal transplantation.[2] CHC is also responsible for decreased graft and patient survival in patients with CKD who undergo kidney transplantation with HCV infection.[2] Due to the risk of allograft rejection, the use of interferon is contraindicated in post renal transplant patients except in emergent situations like fibrosing cholestatic hepatitis (FCH) and life threatening vasculitis; hence the treatment of patients with ESRD and CHC is feasible only prior to kidney transplant.[3]
Overall, this is a difficult group to treat and is associated with poor tolerance of interferon, higher complications and higher dropout rate which may ultimately affect the treatment outcome.[4,5] The aim of our study was to analyze the treatment outcome in patients with CHC and end stage renal disease (ESRD), who were being evaluated for kidney transplant.
Methods
We retrospectively analyzed the data of 65 ESRD with CHC patients, waiting for a renal transplant and visiting our Institute’s Liver Clinic, over a period of two and a half years. The study was approved by our Institute Ethics Committee. The diagnosis of CHC was based on positive HCV RNA by RTPCR, with or without positive antibodies to HCV (anti-HCV, 3rd generation ELISA), and with or without elevated serum alanine aminotransferase (ALT) levels. Patients with liver cirrhosis were excluded from the study. During the initial visit, all patients were explained about their disease and possible treatment and were advised basic work-up including complete hemogram, liver function tests and a repeat testing for anti-HCV antibody and/ or HCV RNA and hepatitis B surface antigen (HBsAg). Quantitative HCV RNA viral load and HCV genotypes were evaluated only in patients willing for treatment. Patients willing for treatment were also screened for baseline autoimmune markers and thyroid function tests. Liver biopsy was advised in patients where advanced fibrosis or cirrhosis could not be excluded by blood investigations, imaging and upper gastrointestinal endoscopy. Duration and type of dialysis was recorded for all patients. Patients willing for treatment were treated with either injection pegylated interferon (peg IFN) in adosage of 1µg/kg body weight (peg a2b) or a fixed dosage of 135 µg (peg a2a) given subcutaneously weekly or with
injection conventional interferon (2a or 2b), 3 million units given subcutaneously three times a week. None of the patients were given ribavirin. All patients were on variable doses of injection erythropoietin for CKD associated anemia (mean hemoglobin: 9.1±2.1 g/dl). Patients were monitored for various complications of interferon treatment and treatment response was assessed by an undetectable HCV RNA for rapid virological response (RVR), complete early virological response (EVR), end of treatment response (ETR) and sustained virological response (SVR) at 4 and 12 weeks, at end of treatment at (24 weeks or 48 weeks depending upon the viral genotype) and 24 weeks after the end of treatment, respectively. Treatment was discontinued in patients not achieving complete EVR (i.e. not achieving negative HCV RNA at 12 weeks).
Results
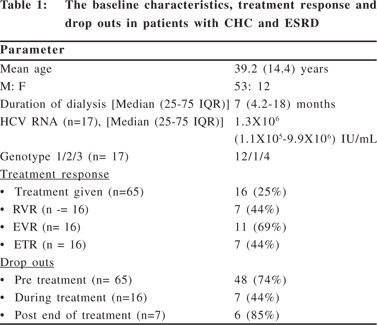
There were 65 patients (males: 53, mean age 39.2±14.4 years) with ESRD and CHC who visited the Liver Clinic during the study period and were diagnosed as CHC during evaluation for kidney transplant. All patients were receiving hemodialysis (HD), with the duration of dialysis varying from 1–60 months (Table 1). All but 4 patients were diagnosed with CHC while they were on dialysis rather than being picked up at screening before starting HD. All patients were HCV RNA positive with 21 patients (32%) only being positive for HCV RNA and negative for anti-HCV antibodies. Quantitative HCV RNA and HCV genotyping was advised in 17 (26%) patients who came back with the initial work-up and were willing for treatment. Other 48 (74%) patients did not come back with the initial baseline work up and were assumed to be unwilling for treatment. Liver biopsy was done in only one patient. The predominant viral genotype amongst treated patients was genotype 1 (Table 1). One patient later decided to get treatment at another center and 16 patients were treated at our center as outpatients. Thirteen patients were treated with peg IFN and three with conventional IFN. The RVR at 4 weeks was achieved in 7 patients (44%) and EVR at 12 weeks was achieved in 11 (69%) patients. In two patients the treatment had to stopped before 12 weeks due to worsening anemia (one patient) and hepatic decompensation in the form of ascites (one patient).
One patient was lost to follow-up before completion of 12 weeks. The treatment was stopped at 12 weeks in two patients who did not achieve EVR. Of the 11 patients who achieved EVR, ETR was achieved in 7 (44%) of them. Two patients were lost to follow-up before end of treatment and two patients stopped their treatment at 18 weeks for not being able to afford their medications. There was no difference in the RVR, EVR or ETR with respect to the viral genotypes infecting the patients. An SVR at six months could be demonstrated in only one of the seven patients who waited for six months after ETR. Other six patients were lost to follow-up after the end of treatment. Follow-up data after kidney transplantation was not available for any patient.
 Discussion
Inadequate infection control is responsible for a high prevalence (12- 40%) of CHC in CKD patients in India.[1] Studies from the Asia-Pacific on HCV amongst 2,01,590 dialysis patients (HD, n = 1,73,788; PD, n = 27,802), revealed an anti-HCV positivity in 0.7-18% of the patients, with a very high prevalence in India.[6] In a study on 256 patients (118 on HD, 138 post-renal transplant) from south India, anti-HCV positivity was seen in 46% and co-infection with HBV was noted in 37% patients.[7]
Despite the high sensitivity of third generation ELISA for anti- HCV testing, the prevalence figures might be an underestimate, since many of these patients are anti-HCV negative and positive only for HCV RNA.[8] More than one-third of our patients with ESRD were also negative for anti-HCV antibodies and were detected only on HCV RNA testing. We believe that the latter should be the standard of care for screening HCV in ESRD patients awaiting a kidney transplant, especially in high prevalence settings like those seen our country.[2] Even though genotype 3 is the commonest HCV genotype in our non-CKD patients, we observed that genotype 1 was more common in our ESRD patients. Reasons for this observation are not clear but similar data has been reported earlier from India.[9]
CHC in the setting of CKD is a difficult sub-group to treat, with outcome depending upon whether the patients are able to complete the treatment or not. Unfortunately in a country like ours with limited resources, not all patients with ESRD and CHC are able to initiate treatment because of their inability to afford the medications, and higher dropout rate is noted in these patients.[1,10] The steep costs of repeated dialysis, medications for ESRD and preparation for a renal transplant, tend to impose a significant financial strain on ESRD patients. Thus the added expenditure of HCV testing and treatment, which may go on for 24-48 weeks, further prolonging dialysis requirements, can be a major challenge for these patients. Hence many of these patients when explained about the length and outcome of HCV treatment and its attended challenges, tend to opt out and either go for a direct renal transplant with their HCV infection or choose an alternative mode of treatment. Forty eight (74%) of our 65 patients perhaps opted this route and did not return after their initial consultation for CHC. Finally only 17 (26%) patients agreed for treatment. This is unlike our experience with non-CKD patients where majority of the patients agree to start treatment and do fairly well on it.[11]
Given the risk of hemolysis and worsening anemia, the standard of care in ESRD patients on dialysis is to use peg IFN or conventional IFN alone without ribavirin, even though there are studies which have used low dose ribavirin in this subgroup of patients as well.[2,3,12] Amongst those who completed the treatment, initial studies achieved better SVR in patients with CKD than non-CKD patients even with conventional IFN.[13]
Discussion
Inadequate infection control is responsible for a high prevalence (12- 40%) of CHC in CKD patients in India.[1] Studies from the Asia-Pacific on HCV amongst 2,01,590 dialysis patients (HD, n = 1,73,788; PD, n = 27,802), revealed an anti-HCV positivity in 0.7-18% of the patients, with a very high prevalence in India.[6] In a study on 256 patients (118 on HD, 138 post-renal transplant) from south India, anti-HCV positivity was seen in 46% and co-infection with HBV was noted in 37% patients.[7]
Despite the high sensitivity of third generation ELISA for anti- HCV testing, the prevalence figures might be an underestimate, since many of these patients are anti-HCV negative and positive only for HCV RNA.[8] More than one-third of our patients with ESRD were also negative for anti-HCV antibodies and were detected only on HCV RNA testing. We believe that the latter should be the standard of care for screening HCV in ESRD patients awaiting a kidney transplant, especially in high prevalence settings like those seen our country.[2] Even though genotype 3 is the commonest HCV genotype in our non-CKD patients, we observed that genotype 1 was more common in our ESRD patients. Reasons for this observation are not clear but similar data has been reported earlier from India.[9]
CHC in the setting of CKD is a difficult sub-group to treat, with outcome depending upon whether the patients are able to complete the treatment or not. Unfortunately in a country like ours with limited resources, not all patients with ESRD and CHC are able to initiate treatment because of their inability to afford the medications, and higher dropout rate is noted in these patients.[1,10] The steep costs of repeated dialysis, medications for ESRD and preparation for a renal transplant, tend to impose a significant financial strain on ESRD patients. Thus the added expenditure of HCV testing and treatment, which may go on for 24-48 weeks, further prolonging dialysis requirements, can be a major challenge for these patients. Hence many of these patients when explained about the length and outcome of HCV treatment and its attended challenges, tend to opt out and either go for a direct renal transplant with their HCV infection or choose an alternative mode of treatment. Forty eight (74%) of our 65 patients perhaps opted this route and did not return after their initial consultation for CHC. Finally only 17 (26%) patients agreed for treatment. This is unlike our experience with non-CKD patients where majority of the patients agree to start treatment and do fairly well on it.[11]
Given the risk of hemolysis and worsening anemia, the standard of care in ESRD patients on dialysis is to use peg IFN or conventional IFN alone without ribavirin, even though there are studies which have used low dose ribavirin in this subgroup of patients as well.[2,3,12] Amongst those who completed the treatment, initial studies achieved better SVR in patients with CKD than non-CKD patients even with conventional IFN.[13]
This was related to the prolonged half-life of interferon, relatively recent onset of disease, mild liver histology and low viral loads in CKD patients. Even though encouraging data on the use of peg IFN in patients with CKD is emerging, peg IFN was poorly tolerated and was associated with substantial side effects in patients with CKD and CHC.[14,15] Though our study had more patients treated with peg IFN, the cost of treatment in these patients can be brought down with the use of conventional IFN. There is data to suggest that the response rates for CHC are no different with conventional IFN and peg IFN, in the setting of CKD. In a recent meta-analysis [20 studies (459 patients) – conventional IFN treated and 2 studies (49 patients) – peg IFN treated], an SVR was achieved in 41% (CI 33-49) and 37% (CI 9-77) of patients treated with conventional IFN and peg IFN, respectively. Treatment discontinuation was also similar with both types of IFNs (26% - conventional IFN, 28% - peg IFN).[16] Other than the convenience of once weekly dosage, peg IFN may not really add any other advantage in this sub-group of patients.
An absence of EVR reasonably predicts a lack of future SVR in patients with CHC.3 One-third of our patients (31%) did not achieve an EVR and were thus unlikely to achieve an SVR.On the other hand, of the 11 patients who achieved an EVR, an ETR was achieved in 7 of these patients (64%). Even though our end of treatment response rate (44%) is less than that in non-CKD patients, it is still acceptable and may improve with increasing number of patients.11 Though it is always advisable to follow patients with CHC and look for an SVR 24 weeks after the end of treatment; in patients with CKD and CHC, kidney transplantation can be done one month after the end of treatment rather than waiting for another 24 weeks.2 We had SVR data available only in one patient and six patients who were lost to follow-up presumably underwent kidney transplantation after achieving their ETR.
Even though our study has the inherent limitation of its retrospective design, we observed a very high rate of patient dropout before, during and after treatment in patients with CHC and ESRD. Maximum patients in our study dropped out before the start of treatment, when 48 of 65 patients (74%) did not agree for it. Inability to afford medications, choosing an alternative mode of treatment or undergoing kidney transplantation with HCV infection, are the probable reasons for this attrition. Seven patients (44%) dropped out during treatment (3 before 12 weeks and 4 after 12 weeks). The reasons for dropout during treatment were mainly related to increasing expense in 4 patients (25%) and intolerance or side effects of drugs in two patients (13%). Six patients were also lost to followup after the end of treatment. Despite the high dropout rate, 44% of our patients (7 of 16 treated) could achieve end of treatment response which suggests a reasonable outcome if these patients start treatment and adhere to it. Since the only chance of successful treatment of CHC in ESRD patients is prior to their kidney transplant, it becomes imperative for the care-giver to adequately motivate these patients by informing them of complications like liver cirrhosis, FCH, poor graft and patient survival, in case a renal transplant is taken up with the HCV infection untreated.[17,18] Since the treatment is difficult in these patients, prevention of HCV infection even by adopting an ‘isolation’ policy is justified in these patients.[19,20]
In conclusion, our retrospective study suggests that treatment of CHC in Indian patients with ESRD is difficult and patient dropout before, during and after treatment is a major problem. Of those who complete treatment, around half of them are able to achieve the end of treatment response. Prospective studies with larger number of patients are required to substantiate our findings.
References
- Agarwal SK, Dash SC, Irshad M. Hepatitis C virus infection during haemodialysis in India. J Assoc Physicians India. 1999;47:1139–43.
- Kidney Disease: Improving Global Outcomes (KDIGO) et al. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;108:S1–99.
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74.
- Berenguer M. Treatment of chronic hepatitis C in hemodialysis patients. Hepatology. 2008;48:1690–9.
- Rendina M, Schena A, Castellaneta NM, Losito F, Amoruso AC, Stallone G, et al. The treatment of chronic hepatitis C with peginterferon alfa-2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768–74.
- Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, Han DS, et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24:1598–603.
- Chandra M, Khaja MN, Hussain MM, Poduri CD, Farees N, Habeeb MA, et al. Prevalence of hepatitis B and hepatitis C viral infections in Indian patients with chronic renal failure. Intervirology. 2004;47:374–6.
- Lakshmi V, Reddy AK, Dakshinamurty KV. Evaluation of commercially available third-generation anti-hepatitis C virus enzyme-linked immunosorbent assay in patients on haemodialysis. Indian J Med Microbiol. 2007;25:140–2.
- Seth AK, Chandra A, Puri P, Kaur J. Genotype 1 hepatitis C virus infection predominates among patients with chronic kidney failure and renal allograft recipients in India. Indian J Gastroenterol. 2009;28:159–60.
- Agarwal SK, Dash SC, Irshad M, Gupta S, Bhowmik D, Tiwari SC, et al. Impact of hepatitis C virus infection on renal transplant outcome in India—a single centre study. J Assoc Physicians India. 2000;48:1155–9.
- Tohra SK, Taneja S, Ghosh S, Sharma BK, Duseja A, Dhiman RK, et al. Prediction of sustained virological response to combination therapy with pegylated interferon alfa and ribavirin in patients with genotype 3 chronic hepatitis C. Dig Dis Sci. 2011;56:2449–55.
- Carriero D, Fabrizi F, Uriel AJ, Park J, Martin P, Dieterich DT. Treatment of dialysis patients with chronic hepatitis C using pegylated interferon and low-dose ribavirin. Int J Artif Organs. 2008;31:295–302.
- Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Metaanalysis: interferon for the treatment of chronic hepatitis C in dialysis patients. Aliment Pharmacol Ther. 2003;18:1071–81.
- Amarapurkar DN, Patel ND, Kirpalani AL. Monotherapy with peginterferon alpha-2b {12 kDa} for chronic hepatitis C infection in patients undergoing haemodialysis. Trop Gastroenterol. 2007;28:16–8.
- Russo MW, Ghalib R, Sigal S, Joshi V. Randomized trial of pegylated interferon á-2b monotherapy in haemodialysis patients with chronic hepatitis C. Nephrol Dial Transplant. 2006;21:437–43.
- Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263–77.
- Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:1452–61.
- Seth AK, Anand AC, Gedela SR, Varma PP, Baliga KV. Rapid progression of hepatitis C-induced liver failure in renal allograft recipients. Indian J Gastroenterol. 2006;25:155–6.
- Agarwal SK, Dash SC, Gupta S, Pandey RM. Hepatitis C virus infection in haemodialysis: the ‘no-isolation’ policy should not be generalized. Nephron Clin Pract. 2009;111:c133–40.
- Agarwal SK. Hemodialysis of patients with HCV infection: isolation has a definite role. Nephron Clin Pract. 2011;117:c328–32.
|
|
|
 |
|
|