48uep6bbphidvals|386
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Helicobacter pylori is a microaerophilic spiral-shaped, gramnegative bacillus which was discovered more than two decades ago by Warren and Marshall.[1] These organisms colonize approximately half of the world’s human population.[2,3] It is considered to play a major role in the pathogenesis of several gastroduodenal diseases, including gastric ulcer, duodenal ulcer, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and distal gastric cancer.[4] Although accurate noninvasive methods such as the urea breath test, the stool antigen test, and serology are available, biopsy based invasive techniques including the rapid urease test, histology and culture, are required to confirm the infection.[5] Moreover, isolation of H. pylori from gastric mucosal biopsy specimens is important to confirm H. pylori as the causative agent of gastritis and is a prerequisite for further studies of the organism addressing drug susceptibility testing, analysis and characterization of virulence factors, molecular epidemiology studying or other comparative studies.[6] Many hospitals and research laboratories have found it difficult to culture H. pylori from gastric mucosal biopsy specimens.[6] The isolation rates of H. pylori from the infected individuals vary depending on a number of factors, including, biopsy preparation, transport and culture media, and the isolation methods used. It has been reported to be within 23.5% to 97% in different studies[7,8]. Due to specific growth requirements, and slow growth (3–5 days), isolation of this microorganism remained difficult.[9,10]
In this study, we aimed to evaluate two different culture medias to establish the most appropriate conditions for isolation of H. pylori strains from clinical samples.
Methods
Study Design
Between October 2008 and October 2009, 266 dyspeptic patients attending the endoscopy ward of Motahhary Clinic of Shiraz University of Medical Sciences, were investigated. Exclusion criteria for patients recruited to the study were previous attempts to eradicate H. pylori and use of antibiotics or proton pomp inhibitors within the last 2 months prior to endoscopy,and previous gastric surgery. The diagnosis of H. pylori infection and the confirmation of gastric disease by histology were established by a pathologist. Two antral biopsies were taken from each patient. The biopsies were put into a tube containing transfer media (Brain Heart Infusion broth supplemented with 20% glucose) and sent to the laboratory to be cultured for H. pylori as soon as possible (within 3hrs). The research protocol was approved by the relevant institutional review board and all the patients had given written informed consent.
Isolation and identification of H. pylori
Each biopsy was divided into three parts. One part was immediately transferred to a tube containing rapid urease test medium. The other part was scraped on a slide to prepare smear.
A modified Gram stain with carbol fuchsin (1:10) was used for staining the smears. The third part of the biopsy was directly put on the selective media and scraped completely on the surface of the plates using a sterile loop. Here, we used 2 selective media; Brucella agar base (Merck, Germany), supplemented with 10% lysed horse blood and : amphotericin B (2 mg/L), trimethoprim (5 mg/L) and nalidixic acid (10 mg/L) (Sigma Chemical Co) (M1 medium), which we used in our previous studies,[11] and a modified medium containing Colombia agar base (Merck, Germany) supplemented with 10% lysed horse blood, 0.25% yeast extract, 7% fetal calf serum (Gibco, USA) and amphotericin B (5 mg/L), trimethoprim (5 mg/L) and vancomycin (10 mg/L) (M2 medium). The cultures were kept in a microaerophilic atmosphere (7% O2, 7.1% Co2, 7.1% H2, 79.8% N2), provided by Anoxomate (Mark ÉÉ, Mart Microbiology BV, Netherlands) at 37 °C. The plates were inspected first on day 1 and then on daily basis for a total of 10 days. The isolates were confirmed as H. pylori by colony morphology and positive oxidase, catalase and rapid urease tests. We used the same media and culture conditions to subculture the isolates for several times. Specimens were considered to be H. pylori positive if either the culture or two of the three diagnostic methods yielded positive results.
Preservation of isolates and recovery
The isolates were collected from the surface of the media after 48 hrs, were subcultured and preserved in cryo tubes in an enriched medium; Skim Milk (Merck, Germany), supplemented with 10% fetal calf serum (Gibco, USA) and 15% glycerol. The bacteria collected from the surface of one full growth medium, were inoculated in a tube containing 500 µl preservative medium. The tubes were then kept at -70 °C. After 4 weeks, 6 months, and 10 months for each initial culture medium, 10 isolates were thawed at room temperature and inoculated on the surface of the respective selective medium. Incubation and identification of the strains were carried out as for primary isolation.
Statistical Analysis
Statistical analysis was performed using SPSS software for Windows, version 11.5 (SPSS). Chi-Square (X2) test and p values were determined. P value of <0.05 was considered significant.
Results
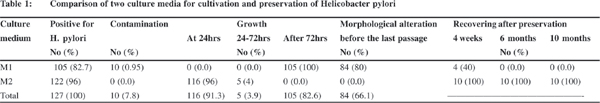
In total, 127 specimens were positive for H. pylori, of which 122 (96%) and 105 (82.7%) strains were isolated using M2 and M1 medium, respectively ( p<0.05) (Table 1). All strains isolated Isolation and preservation of clinical strains of H. pylori 37 on M2 medium were pure and without any contamination, while ten strains obtained by using M1 medium had fungal or bacterial contaminations. H. pylori colonies were larger on M2 than M1 medium and 96% of the isolates appeared after 24 hours, while bacterial growth on M1 was observed after a significantly longer time (p<0.05), i.e., after incubation for about 3 days in 80% of the isolates. The other 20% of the isolates appeared after more than 4 days. On M2 medium, 95% of the all strains survived more than 5 subcultures, and 7 of 10 randomly selected isolates survived more than 9 subcultures. No significant morphological alteration or transformation of cells to coccoid forms was observed in gram staining of culture before each passage. However, 85% of the isolates survived up to 3 subcultures on M1 medium and the remaining isolates could be subcultured up to 4 times. Before the last passage on M1 medium, about 80% of the bacterial cells had a morphological alteration from bacilli to coccoid form in the gram staining. Preservation of the strains isolated from M2 kept all 10 (100%) strains viable after 4 week, 6 month or 10 month period of storage at -70 oC. Only 4 from 10 (40%) of the strains isolated from M1, could be recovered after 4 weeks of the storage in preservative condition.
Discussion
H. pylori, as a fastidious organism requires an enriched media with an appropriate microaerophilic condition. The success in culture and isolation of this organism was obtained in 1984.[12] Culture has been considered the gold standard in confirmation of the diagnosis of H pylori infection.[5] Moreover, isolation of H. pylori from gastric mucosal biopsy specimens is a prerequisite for further studies of the organism including drug susceptibility testing, analysis and characterization of virulence factors, molecular epidemiology studying or other comparative studies.[5] Primary isolation of H. pylori from clinical samples is affected by several factors related to the culture condition and biopsy preparation.[13]Collection procedure and transport of the biopsy samples have been found to be a very important factor in the successful growth of this microorganism.[12]
In this study, we tried to improve the culture conditions so fast improve the isolation rate , shorten the duration of the incubation and prolong the life time of preserved organisms. Two enriched media were used to culture the biopsy specimens but with the same conditions related to biopsy preparation and transferring. According to our previous studies and the studies by others, it seems that time taken between collection of the biopsy and inoculation of the specimen on different media is more important in the isolation rate of H. pylori. So, all the samples were transported to the lab in less than 3 hours and in an enriched transfer media. When delay occurred, the specimens were more liable to get contaminated with other bacteria. Moreover, H. pylori cells may transform from a cultivable spiral-shaped form to a non-cultivable coccoid form, in which the recovery of the bacterium is very difficult by routine culture methods.[14] The biopsies were directly put on the selective media and scraped completely on the surface of the plate using a sterile loop. This method was found to be superior to rubbing the biopsy on culture plates, scraping the biopsy between the slides or crushing in a tissue grinder because H.pylori organisms are very sensitive to the dryness which may occur during the process of crushing, and consequent recognition of the microorganism by gram staining is difficult due to background material. Using fresh media made with fresh blood and maintenance of adequate humidity throughout incubation are necessary for successful isolation of H. pylori, variations have been described with respect to the composition of the culture medium, and atmospheric conditions.[15,16,17] Also, considering the high rate of contaminations on nonselective media, use of antibiotics is thought to be mandatory for the detection of H. pylori.[9,18] Brucella agar, brain heart infusion, chocolate agar, colombia agar, containing either 5 or 10% sheep blood[9,12,19,20,21] or 7% horse blood[20,22,23] egg yolk emulsion,[24] cyclodextrins[25] and modified Thayer-Martin medium[26,27] have been used successfully to isolate H. pylori strains. In all these studies the incubation period for cultivation was from 3 to 12 days. The problem facing the prolonged incubation times is overgrowth contaminants especially fungal agents, even in the presence of anti fungal or antibacterial drugs. Nalidixic acid has also been suggested to inhibit 14% of H. pylori isolates at a concentration of 20 mg/L.[28] Considering all these factors , in this study we used a modified enriched culture medium to isolate the highest rate of H. pylori from the clinical specimens and in the shortest incubation time, which was 24 hours. Additionally, the cultures were pure and without any contamination.
For long term storage of the clinical isolates, preservation of the strains is necessary. It has been shown that freeze-drying harms H. pylori.[18] On the other hand, lyophilizing without freezing by use of 20% L-glutamic acid solution has been recommended but is too cumbersome for routine usage.[18,29] Based on laboratory experience, different methods have been recommended for the preservation of H. pylori strains, such as storage at -70°C in 1% peptone water containing 25% glycerol,[18] in defibrinated horse blood,[29] in tryptone soy broth containing 15% glycerol, or in a commercial cryopreservative fluid.[30] Most of them have a low rate of recovering of the live organism after a 6 month period of storage. In this study, 10 fresh isolates which were cultivated on two kinds of enriched media, M1 and M2, were preserved in an enriched preservative medium, containing skimmed milk supplemented with 10% fetal calf serum and 15% glycerol at -70 oC. All the strains (100%) recovered from M2 were viable for a long time up to 10 month period of storage, while only 40% of the strains isolated from M1 could be recovered after 4 weeks of the storage in the same preservative condition. This data indicates the importance of the culture condition for the initial isolation of the organisms. It is obvious that if bacteria could be maintained in a good condition during the first cultivation just before preservation, the chance for recovering them in viable form will be greater. Moreover, following these procedures, the preservation time could be extended beyond 6 months without a significant loss of viability.
In conclusion, as culture is important to the study of the characteristics and antibiotic sensitivity pattern for the treatment of infections caused by H. pylori, the modified culture technique enabled a shorter incubation time and a higher isolation rate for H.pylori obtained from clinical samples. This also enabled the isolate to preserved for a longer time.
References
1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5.
2. Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–41.
3. Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31.
4. Farshad Sh, Japoni A, Alborzi A, Zarenezhad M, Ranjbar R. Changing prevalence of Helicobacter pylori in south of Iran. Iran. J Clin Infec Dis. 2010;5:65–9.
5. Yin Y, He LH, Zhang JZ. Successful isolation of Helicobacter pylori after prolonged incubation from a patient with failed eradication therapy. World J Gastroenterol. 2009;15:1528–9.
6. Hachem CY, Clarridge JE, Evans DG, Graham DY. Comparison of agar based media for primary isolation of Helicobacter pylori . J Clin Pathol. 1995;48:714–6.
7. Fresnadillo Martinez MJ, Rodriguez Rincon M, Blazquez de Castro AM, Garcia Sanchez E, Garcia Sanchez JE, Trujillano Martin I, et al. Comparative evaluation of selective and nonselective media for primary isolation of Helicobacter pylori from gastric biopsies. Helicobacter. 1997;2:36–9.
8. Heep M, Scheibl K, Degrell A, Lehn,N. Transport and storage of fresh and frozen gastric biopsy specimens for optimal recovery of Helicobacter pylori. J Clin Microbiol. 1999;37:3764–6.
9. Buck G, Smith JS. Medium supplementation for growth of Campylobacter pyloridis. J Clin Microbiol 1987;25:597–9.
10. Price AB, Levi J, Dolby JM, Dunscombe PL, Smith A, Clark J, et al. Campylobacter pyloridis in peptic ulcer disease: microbiology, pathology and scanning electron microscopy. Gut. 1985;26:1183–8.
11. Farshad S, Japoni A, Alborzi A, Hosseini M. Restriction fragment length polymorphism of virulence genes cagA, vacA and ureAB of Helicobacter pylori strains isolated from Iranian patients with gastric ulcer and nonulcer disease. Saudi Med J. 2007;28:181–86.
12. Rizvi F, Hannan A. Evaluation of different transport and enrichment Media for the isolation of Helicobacter pylori. JAMC. 2000;12:31–4.
13. van der Hulst RW, Verheul SB, Weel JF, Gerrits Y, ten Kate FJ, Dankert J, et al. Effect of specimen collection techniques, transport media, and incubation of cultures on the detection rate of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1996;15:211– 5.
14. Shahamat M, Alavi M, Watts JE, Gonzalez JM, Sowers KR, Maeder DW, et al. Development of two PCR-based techniques for detecting helical and coccoid forms of Helicobacter pylori. J Clin Microbiol. 2004;42:3613–9.
15. Boyanova L. Influence of transport conditions and media on Helicobacter pylori isolation. J Med Microbiol. 2003;52:1129– 30.
16. Vega AE, Cortinas TI, Mattana CM, Silva HJ, Puig de Centorbi O. Growth of Helicobacter pylori in medium supplemented with cyanobacterial Extract. J Clin Microbiol. 2003;41:5384–8.
17. Perez-Perez GI. Accurate Diagnosis of Helicobacter pylori. Culture, including transport. Gastroenterol. Gastroenterol Clin North Am. 2000;29:879–84.
18. Goodwin CS, Armstrong JA. Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur J Clin Microbiol Infect Dis. 1990;9:1–13.
19. Taylor DE, Hargreaves JA, Ng LK, Sherbaniuk RW, Jewell LD. Isolation and characterization of Campylobacter pyloridis from gastric biopsies. Am. J Clin Pathol. 1987;87:49–54.
20. Graham DY, Klein PD, Evans DJ Jr, Evans DG, Alpert LC, Opekun AR, et al. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet. 1987;1:1174–7.
21. Krajden S, Bohnen J, Anderson J, Kempston J, Fuksa M, Matlow A, et al. Comparison of selective and nonselective media for recovery of Campylobacter pylori from antral biopsies. J Clin Microbiol. 1987;25:1117–8.
22. Marshall BJ, Royce H, Annear DI, Goodwin CS, Pearman JW, Warren JR, et al. Original isolation of Campylobacter pyloridis from human gastric mucosa. Microbios Lett. 1984;25:83–8.
23. Hazell SL, Lee A, Brady L, Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986;153:658–63.
24. Westblom TU, Madan E, Midkiff BR. Egg yolk emulsion agar, a new medium for the cultivation of Helicobacter pylori. J Clin Microbiol 1991;29:819–21.
25. Olivieri R, Bugnoli M, Armellini D, Bianciardi S, Rappuoli R, Bayeli PF, et al. Growth of Helicobacter pylori in media containing cyclodextrins. J Clin Microbiol. 1993;31:160–2.
26. Glupczynski Y, Labbe M, Thibaumont F. Comparative evaluation of a new selective culture medium for improved isolation of Campylobacter pylori from gastric biopsy specimens, In: Megraud F, Lamouliatte H, editors. Gastroduodenal pathology and Campylobacter pylon. New york: Elsevier; 1989.p.3–6.
27. Parsonnet J, Welch K, Compton C, Strauss R, Wang T, Kelsey P, et al. Simple microbiologic detection of Campylobacter pylori. J Clin Microbiol. 1988;26:948–9.
28. Dent JC, McNulty CAM. Evaluation of a new selective medium for Campylobacter pylori. Eur J Clin Microbiol Infect Dis. 1988;7:555–68.
29. Westblom TU, Barthel JS, Havey AD, Gonzalez FJ, Tarka EF, Everett E. Long term freeze storage of Campylobacter pyloridis. J Clin Pathol. 1987;40:353.
30. Ribeiro CD, Gray SJ. Long term freeze storage of Campylobacter pyloridis. J Clin Pathol. 1987;40:1265.