48uep6bbphidvals|362
48uep6bbph|2000F98CTab_Articles|Fulltext
offshoreheight: normal; margin: 0in 0in 0pt;">Introduction
Hepatocellular carcinoma (HCC) is the commonest primary cancer of the liver. Its incidence is increasing and has risen to become the 5th commonest malignancy worldwide and the third leading cause of cancer related death, exceeded only by cancers of the lung and stomach.[1] In India, the mean incidence of HCC in four population-based registries is 2.77% for males and 1.28% for females. The prevalence of HCC in India varies from 0.2%to 1.6%.[2,3] Despite India being a low incidence zone for HCC, the estimated number of HCC patients in 2001 were 12750.[2] In the West, majority of HCC are diagnosed incidentally during routine evaluation. However, in India, most of the patients in clinical practice present at an advanced stage ruling out curative treatment in most patients.
In recent years, great progress has been made in the diagnosis and management of HCC; high-risk groups for this disease are now recognized and the number of patients with resectable HCC and small-sized HCC is increasing because of better screening strategies, improved surgical techniques, and the development of alternative treatments. Accordingly several new clinical staging systems for HCC have been developed in recent years.[4,5,6,7,8,9,10,11,12]
The staging systems most often used are: Okuda staging, the Cancer of the Liver Italian Program (CLIP), the Barcelona Clinic Liver Cancer staging (BCLC), the Japan Integrated Staging Score (JIS), the Chinese University Prognostic Index, the French Score and the Tokyo score.[4,5,6,7,8,9,10,11,12] There are myriad studies which compare these staging systems for predicting survival in HCC patients. However there is no report from India which compares these different staging systems in HCC patients, hence this study compares different staging systems in a North Indian cohort with HCC.
Methods
From August 2007 to September 2008, a total of 101 patients, diagnosed with HCC at the Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India were prospectively enrolled in this study. Diagnosis of HCC was made on the basis of:
· Histological confirmation or two concordant imaging studies namely computed tomography (CT), and magnetic resonance (MR) with typical findings of HCC or one imaging study in addition to AFP level >400 ng/ml
For all patients, demographic information, aetiology (HBV, HCV, and alcohol), biochemical and haematological data were recorded. Presence of portal hypertension was assessed by ultrasonography and upper GI endoscopy. Presence of underlying cirrhosis was based on clinical and radiological evidence of portal hypertension or on histology. Presence of ascites on USG and history suggestive of hepatic encephalopathy were also recorded.
In addition the following baseline variables were evaluated:
1. Aetiology of liver disease: Alcohol was considered as an aetiological factor if there was a history of alcohol intake in cirrhogenic doses (> 80 g/d of ethanol for >10 yrs.) with negative viral markers for HBV and HCV. HCC was considered to be HBV or HCV related if HBsAg or Anti- HCV was positive respectively. The etiology was considered as cryptogenic if the etiological work up was negative for HBV, HCV and autoimmune markers with no history of alcohol intake in cirrhogenic doses.
2. Biochemistry and other blood parameters: serum bilirubin, albumin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), creatinine, prothrombin time, international normalized ratio (INR) and AFP (Alpha-Fetoprotein) were assessed in all patients. Serum AFP level was determined by enzyme-linked immunosorbent assay with a commercially available kit (Smart diagnostic, Israel).
3. Se verity of liver disease was based on Child–Pugh score and Model for End Stage Liver Disease (MELD)score.[10, 13,14]
4. Tumor characteristics were assessed by imaging modalities. The maximum diameter of tumor was defined using ultrasonography or other imaging modalities like CT scan/ MRI. The number of tumors and vascular invasion was assessed by ultrasonography and dynamic CT scan. Tumor volume compared to liver volume was assessed by using CT volumetric study.[4] Lymph node invasion and distant metastasis was assessed by routine screening such as ultrasonography, dynamic CT, and chest X-ray. Bone scintigraphy or brain CT was performed if suggestive symptoms were present.
5. Venous obstruction: Portal vein, hepatic vein and inferior vena cava were assessed for patency by ultrasonography, CT scan or MRI.
6. TNM stage developed by LCSGJ (Liver Cancer Study Group of Japan) was determined by tumor characteristics based on imaging studies.[14]
Patients with the above available clinical, laboratory and radiological findings were staged according to different staging systems namely, Okuda classification[11], CLIP scoring system[4], BCLC staging classification[5], JIS score[6], Tokyo score[15], MELD score[13], and GRETCH staging system[12]. Eastern Cooperative oncology Group performance status and Karnofsky index were used in the BCLC and GRETCH staging respectively.[5,12]
The performance of different staging system was assessed by using the survival of HCC patients. Survival was defined as the time between HCC diagnosis, whether at referral centre or at our centre (tertiary care centre) and death (in months) or the last follow up. Follow up was censored on 30th September 2008. Length of survival was calculated from the date of HCC diagnosis to the date of death or, in the case of survivors, the date of the last follow up visit. Only thirty nine (38.6%) patients could be treated. Resection of HCC was possible only in seven (6.9%) patients. Eighteen (17.8%) and twenty four (23.8%) patients had undergone RFA and TACE respectively. Ethical approval for the use of human subjects was obtained from Institutional review board (Ethics Committee).
Statistical Analysis
The data was analyzed using the Statistical Package for Social Sciences (SPSS) version 13.0 (SPSS Inc., Chicago). Descriptive statistics including frequency, mean, median and standard deviation were calculated for the demographic data, hematological and biochemical parameters. The frequencies were derived for the various clinical sign and symptoms, etiological analysis, imaging data including the number of hepatic nodules, their location and size and for different staging systems of HCC. The continuous variables between the two groups were compared by the Mann–Whitney U-test. The Kruskal–Wallis test was used to compare a continuous variable when there were more than two groups. The chi-square test was used to analyze categorical variables between the two groups and the two-tailed Fisher’s exact test where numbers were small. P-value <0.05 was considered to be significant. Univariate survival curves were calculated using the Kaplan–Meier test and were compared by means of the log rank test. The following variables at baseline were considered for univariate analysis: age, gender, Eastern Cooperative Oncology Group – performance status (ECOG – PS), aetiology of cirrhosis (HCV, HBV, alcohol abuse), platelet count, albumin level, bilirubin level, alkaline phosphatase, creatinine levels, international normality ratio (INR), platelet, AFP level, ascites, hepatic encephalopathy, portal vein thrombosis, portal hypertension, cirrhosis, tumour size, number of neoplastic lesions, Child-Pugh class, MELD score and Diabetes mellitus. Variables with a p-value <0.05 at univariate analysis were included in the final multivariate model. Cox’s proportionalhazard model was used to identify prognostic factors for mortality in a multiple regression analysis. All p-values were two-tailed, and all confidence intervals (CIs) were 95%.
Results
Patient characteristics
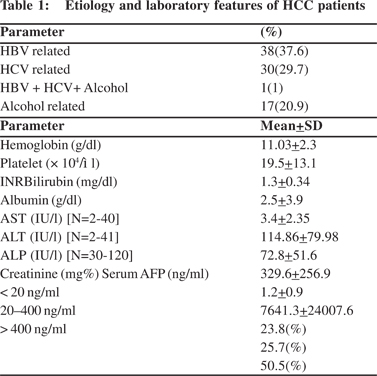
Table 1 lists the etiological, hematological and biochemical profiles of the 101 patients. The majority of the patients were men (93.1%). The median age was 60 years with the youngest patient aged 30 years and the oldest 86 years. The mean age was 58.9 + 11.9 years.
Aetiology and severity of liver disease
The etiological work up revealed HBV in thirty eight (37.6%) patients either alone or as a co-factor with alcohol or HCV (Table 1) and the most common cause associated with HCC. Two HBsAg negative patients were found to be positive both for anti-HBc (total) and HBV DNA.
Clinical features
All the patients had underlying cirrhosis. Thirty eight (37.6%) patients belonged to Childs A. The remaining sixty three (62.3%) patients belonged either to Childs B (46.5%) or Child class C (15.8%). More than half of the patients (65.3%) had a MELD score of >10 indicating poor hepatic functional status.
Radiological features
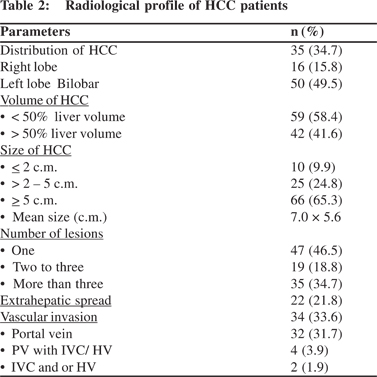
In the study group, number, size, distribution, characteristics, vascular and metastatic details of hepatic nodules were analyzed using USG, CT scan and or MRI abdomen. The radiological profile is presented in Table 2.
Survival and different Staging systems of HCC
At the end of study period, seventy two (71.2%) of 101 patients had died. The median survival was 4 months (range: 1- 55 months). HCC patients were staged according to Okuda, TNM (LCSGJ), BCLC, CLIP, JIS, Tokyo score and GRETCH staging systems.
Okuda stage: Twenty one (20.8%) patients were in Okuda stage I, while fifty nine (58.4%) and twenty one (20.8%) patients were in Okuda stage II and stage III respectively. Median survival was 5, 4 and 4 months in stage I, II and III respectively. There was no significant difference in survival (p=0.33) [Table 3] with the three stages of Okuda.
TNM stage (LCSGJ): Only one patient belonged to stage I and thirty three (32.7 %) patients were in stage II. Majority of the patients (66.4%) were either in stage III or stage IV. The median survival was 5, 5 and 4 months in stages II, III, IV respectively.

There was no significant difference of the staging with survival (p= 0.89) (Table 3).
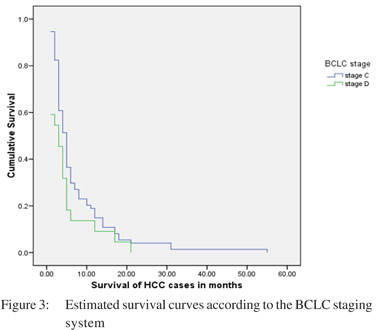
BCLC stage: Most of the patients belonged either to stage C or D. Seventy four (73.3 %) patients were in BCLC stage C and twenty two (21.8%) in stage D. Only five (4.9%) patients belonged to either stage A or B. Median survival was 15, 6, 5 and 3 months in stages A, B, C and D respectively. There was a significant difference of survival between BCLC stage C and D (p= 0.05) (Table 3).
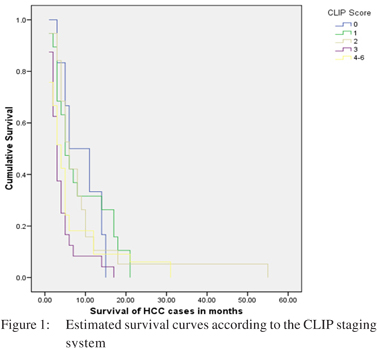
CLIP score: The median CLIP score of HCC patients was 3. Fifty seven (56.4%) patients had a CLIP score of >3. Only six (5.9 %) patients had a zero CLIP score with nineteen (18.8 %) patients each having a CLIP score of 2. The median survival in patients having a CLIP score of <3 was 5.0 months, while those having a score of 4-6 were 4 months. There was a significant difference of survival across the staging system (p=0.03) (Table 3).
JIS score: Fifty four (53.7%) patients had a JIS score of >3 with a median survival of 3 months. Fourteen (13.8%) and thirty three (32.7 %) patients had a score of 1 and 2 respectively with a median survival of 8 and 5 months respectively. There was no significant difference of survival seen with the staging system (p=0.19) (Table 3).
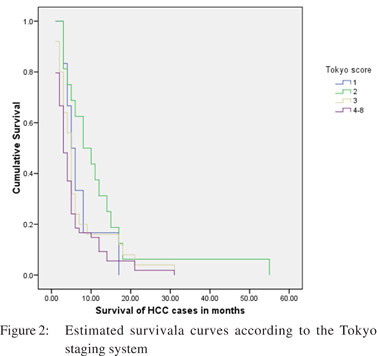
Tokyo score: Fifty four (53.7%) patients had a Tokyo score of >4. Only twenty two (21.7%) patients had a score either of 1 or 2. Twenty five (24.7 %) patients had a score of 3. The median survival in patients having a score of 1 and 2 was 5 and 8 months respectively. Like BCLC staging and CLIP score, this staging also demonstrated a significant difference of survival with a p value of 0.05 (Table 3).
GRETCH stage: Twelve (11.9%) patients were in group A with fifty (49.5%) and thirty nine (38.6%) patients in group B and group C respectively. The median survival in group A, B, C was 6, 5 and 8 months respectively. This staging also failed to demonstrate any significant difference with survival with a p value of 0.29 (Table 3).
CLIP, Tokyo score and BCLC staging system showed a significant difference in the probability of survival according to the Kaplan-Meier method. All other staging systems failed to show a significant difference in survival (Table 3). The survival curves for these staging systems are shown in Figures: 1, 2 and 3.
The BCLC staging in our population mostly ranged from C to D (Table 3). Significant survival difference was found only between stage C and stage D HCC (C vs. D, p=0.05) (Table 3).
Simple predictors of survival
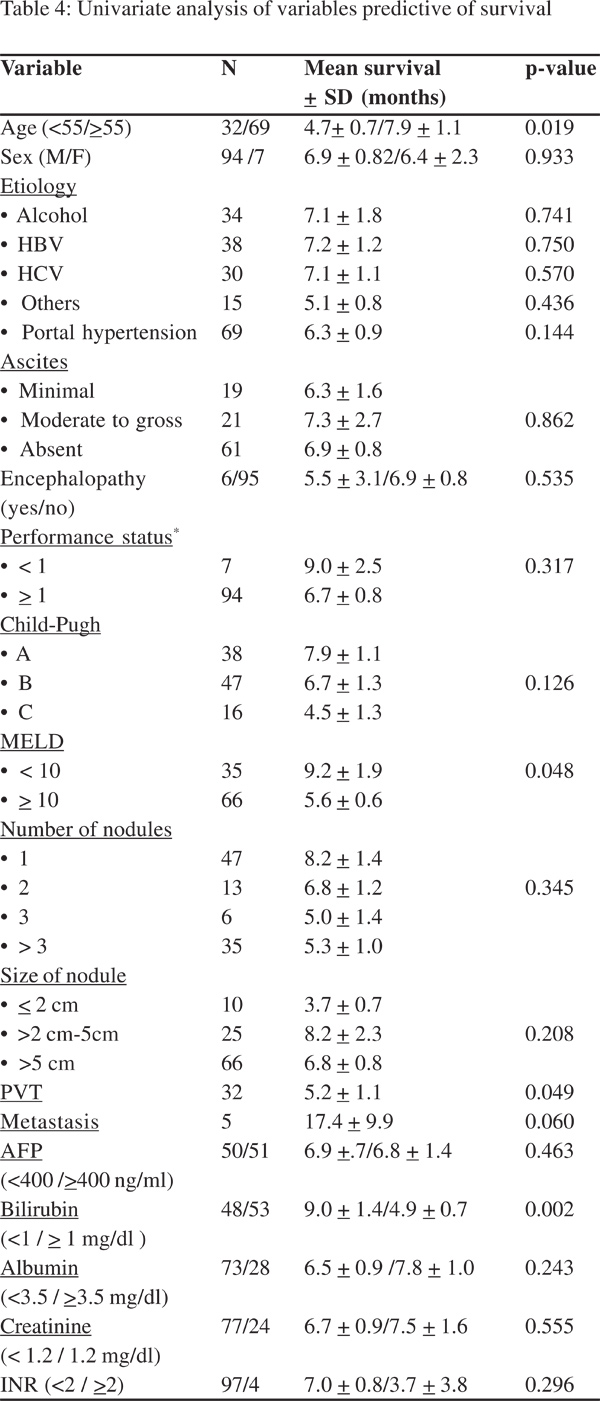
Using the Kaplan-Meier method, we evaluated possible correlations between survival and 25 variables with known values for all 101 patients (Table 4). The four factors that had a significant difference with survival in the log rank test were: age >55 years, presence of PVT, serum bilirubin >1.0mg/dl, MELD score >10 (Table 4).
Multivariate analysis
Variables namely, age >55 years, presence of PVT, serum bilirubin >1.0mg/dl, MELD score >10 and metastasis were included in the final multivariate model. Multivariate analysis revealed that serum bilirubin was an independent predictor of survival with a hazard ratio of 1.609 (95% CI 1.015-2.553, p=0.043).
Discussion
Many prognostic staging systems have been proposed in the last 20 years for HCC, but there is no worldwide consensus on which is the best staging system.[4,5,6,7,8,9,10,11,12] The main factor for the lack of reproducibility is that these staging systems were made on the basis of different patient populations, tumor characteristics and treatment strategies. There are many studies which have evaluated the performance of these different staging systems from all over the world.[9,10,15,16,17,18,19,20,21] However there is no study which compares these different staging systems in Indian population. Hence we assessed the utility of 7 different staging systems in Indian patients with HCC; analysis of independent predictors of survival and their correlation with survival.
In this study, HCC patients were staged according to Okuda, TNM (LCSGJ), BCLC, CLIP, JIS, Tokyo score and GRETCH staging system. All these staging systems showed that most of our patients presented in very advanced stage. Moreover the size of HCC was >5 cm in sixty six (65.3%) patients. The presence of PVT in patients with HCC has been found to be associated with reduced survival.[22,23] In the present study, main trunk of portal vein or its major branches was involved in thirty two (31.7%) patients. Similar results were seen in the study by Sarin et al, though in their study staging of HCC was limited to TNM and Okuda stage.[24] Past studies have shown that HCCpatients with a high AFP concentration (>400 ng/mL) tend to have greater tumor size, bilobar involvement, massive or diffuse types, portal vein thrombosis, and a lower median survival rate.[25,26] Our study did not show any significant correlation of serum AFP with size of tumor.
In this study, at the time the data was censored, 72 of total 101 patients (71.2%) had died. The overall median survival was 4 months (range: 1- 55 months). In a study comprising 239 American patients, at the end of study period, 63% patients had died. The overall median survival was 16.4 months (95% CI 12.9-19.8 months).[9] In the present study, univariate analysis showed age >55 years, presence of PVT, serum bilirubin > 1.0mg/dl, MELD score >10 were significant baseline predictors of survival. At multivariate analysis only serum bilirubin was found to be an independent predictor of survival. All our patients had underlying cirrhosis; hence it is not surprising that survival was related to serum bilirubin. In a study by Camma et al, on multivariate analysis, bilirubin, AFP and portal vein thrombosis were found to be independent predictors of survival.[19]
In a study by Nanashima et al, all the seven staging systems namely Okuda, TNM (UNOS), BCLC, CLIP, JIS, GRETCH and Chinese University Prognostic Index (CUPI) staging systems showed a significant difference in the probability of survival across the different stages.[9] In our study, only CLIP, Tokyo score and BCLC staging system showed a significant relationship to survival.
Being a single centre study, the outcome may not be generalized. Moreover, our hospital is a tertiary care centre; patients with advanced tumors are often referred to us as a last resort. So a referral bias cannot be ruled out. Further, due to differences in demographics, etiology of liver disease and proportion of patients with cirrhosis, our results may not apply to HCC patients of different ethnic groups. Above mentioned facts may be considered as drawbacks of our study.
The epidemiological characteristics of our cohort are consistent with that reported in other studies of Indian patients with HCC.[24, 27] Thus CLIP, Tokyo score and BCLC are the most useful staging systems in Indian patients with HCC.
Acknowledgements
This work was financially supported by Indian Council of Medical Research (ICMR)-New Delhi, India.
References
1. World Health Organization Mortality database. [database on the internet]. World Health Organization. C2011. Available from: http://www.who.int/whosis/en
2. National Cancer Registry Programme. Annual report, 1987. New Delhi: Indian Council of Medical Research; 1990.
3. Jayant K, Rao RS, Nene BM, Dale PS. Rural Cancer Registry at Barshi. Report 1988-92. Barshi: Rural Cancer Registry; 1994.
4. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–5.
5. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–37.
6. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207–15.
7. Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system,and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–9.
8. Nanashima A, Morino S, Yamaguchi H, Tanaka K, Shibasaki S, Tsuji T, et al. Modified CLIP using PIVKA-II for evaluating prognosis after hepatectomy for hepatocellular carcinoma. Eur J Surg Oncol. 2003;29:735–42.
9. Nanashima A, Omagari K, Tobinaga S, Shibata K, Sumida Y, Mine M, et al. Comparative study of survival of patients with hepatocellular carcinoma predicted by different staging systems using multivariate analysis. Eur J Surg Oncol. 2005;31:882–90.
10. Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–16.
11. Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28.
12. Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. Groupe d’Etude et de Traitement du Carcinome Hepatocellulaire. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. J Hepatol. 1999;31:133–41.
13. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6.
14. Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer, 2nd English edition. Tokyo:Kanehara; 2003.
15. Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, et al. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419–25.
16. Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Comparison of the usefulness of three staging systems for hepatocellular carcinoma (CLIP, BCLC, and JIS) in Japan. Am J Gastroenterol. 2005;100:1764–71.
17. Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, et al. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445–52.
18. Lu W, Dong J, Huang Z, Guo D, Liu Y, Shi S. Comparison of four current staging systems for Chinese patients with hepatocellular carcinoma undergoing curative resection: Okuda, CLIP, TNM and CUPI. J Gastroenterol Hepatol. 2008;23:1874–8.
19. Chen TW, Chu CM, Yu JC, Chen CJ, Chan DC, Liu YC, et al. Comparison of clinical staging systems in predicting survival of hepatocellular carcinoma patients receiving major or minor hepatectomy. Eur J Surg Oncol. 2007;33:480–7.
20. Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62–75.
21. Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–405.
22. Ikai I, Itai Y, Okita K, Omata M, Kojiro M, Kobayashi K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21–9.
23. Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Austria: aetiological and clinical characteristics atpresentation. Eur J Gastroenterol Hepatol. 2000;12:941–8.
24. Kumar R, Saraswat MK, Sharma BC, Sakhuja P, Sarin SK. Characteristics of hepatocellular carcinoma in India: a retrospective analysis of 191 cases. QJM. 2008;101:479–85.
25. Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302–8.
26. Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of alphafetoprotein and rotein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128–34.
27. Sarin SK, Thakur V, Guptan RC, Saigal S, Malhotra V, Thyagarajan SP, et al. Profile of hepatocellular carcinoma in India: an insight into the possible etiologic associations. J Gastroenterol Hepatol. 2001;16:666–73.