48uep6bbphidvals|340
48uep6bbph|2000F98CTab_Articles|Fulltext
In extra hepatic biliary atresia (EHBA), Kasai described the presence of small channels, which varied in size up to 300µm in diameter, in the porta hepatis and with three dimensional reconstruction studies these ducts were shown to communicate with intrahepatic ducts.[1,2] This has been the basis for anastomosis of a Roux-en-Y loop of jejunum to the porta hepatis, now known as the Kasai’s portoenterostomy procedure.[3] Although, the prognosis of biliary atresia has dramatically improved with the introduction of liver transplantation, the Kasai’s operation is still the first line of surgical treatment.
The use of steroids and cholagogues, such as phenobarbitol and cholestyramine, to enhance bile flow in cholestatic patients has been described.[4,5] Dehydrocholic acid (DHC), in the past, and ursodeoxycholic acid (UDCA), more recently, have been used with the intention of enhancing success in relief of symptoms, reducing intrahepatic fibrosis and improving the outcome of infantile cholestasis states.[6] This communication describes our experience with the use of DHC and UDCA, although at different time periods, in the management of EHBA and attempts to correlate the outcomes with the diameter of ductules at the porta hepatic in these patients.
Material and methods
Patients
Fifty five patients of EHBA who underwent the Kasai’s portoenterostomy formed the basis of this study. They were divided into two groups : retrospective group (patients treated from 1979-1986 and postoperatively administered DHC) and prospective group (patients treated from 1999-2002 and postoperatively administered UDCA). Patients treated between 1987-1998 were excluded from this study because data regarding the duct size at the porta hepatis was not available.
Data regarding age, sex, clinical history, pre and postoperative biochemical liver function tests (LFT), hepatobiliary scintigraphy (HIDA scan) and histopathological assessment of ductal size of patients operated between 1979-1986 were collected from case records.
Management protocol
All preoperative HIDA scans were done after 5 days of priming with phenobarbitone (5mg/kg). The uptake by liver and excretion into the gut was noted. All patients with no gut excretion for more than 24 hours were subjected to minilaparotomy under general anesthesia; preoperative cholangiogram (POC) was performed if a patent gall bladder was present. Cases proven to be EHBA on POC or those with atretic gall bladder and extrahepatic ducts were subjected to Kasai’s portoenterostomy.
Tissue processing
The excised specimen of gall bladder, extra hepatic ducts and tissue from porta hepatis was subjected to light microscopic histopathological examination (HPE); all specimens were examined by the same senior pathologist (SDG). The tissue was fixed immediately in 10% neutral formalin, then embedded in paraffin for 5µm serial sections in a transverse plane. These sections were stained with Mayer’s hematoxylin and eosin, Masson’s trichrome and Van Geison’s stains. An optical micrometer was used to measure the internal diameter of the ducts at the porta hepatis. The ducts were classified as: TypeI : no demonstrable ducts; Type II : <50µm; and Type III : >50µm in accordance with a previously published report.[7]
Postoperative management and assessment
In the retrospective group (n=35) DHC (3-5 mg/kg) and betamethasone (10 drops/day) were administered for 3 months, where all patients in the prospective group (n=20) were administered UDCA (15mg/kg), phenobarbitone (5mg/kg) for one year, and betamethasone (10 drops/day) for 3 months as soon as oral feeds were instituted.
Patients were monitored for clinical outcome, LFT and biliary excretion by HIDA scan in the first six months; biliary excretion was graded as either excretory or non- excretory. Any episode of cholangitis occurring in the first six months postoperatively was recorded.The outcome of patients as measured by LFT and status of biliary excretion was correlated with the ductal size (types I,II,III). The clinical outcome was broadly categorized as follows :
1. Jaundice free survival at 5 years of follow up
2. Initial good response but deteriorated after one year
3. Expired within one year following surgery
Statistical analysis was done by Microsoft SPSS software for Windows. Comparison of ductal size and outcome (LFT, HIDA) in individual groups were done by student ‘t’ test. Comparison of parameters between the groups were done by Mann-Whitney test. Values of p<0.05 were considered significant.
Results
There were 26 male and 9 female patients (M: F – 2.8:1) who were treated post-operatively with DHC and there were 13 male and 7 female patients (M:F = 1.8:1) who were treated postoperatively with UDCA. In the DHC treated sub-set of patients, there were 13 (37.2%) patients with no demonstrable ducts at the porta hepatic whereas in the UDCA treated subset of patients there was only one (5%) patient with no demonstrable ducts. Type II ducts (<50 µm) were seen in 18 (51.4%) patients in the DHC treated sub-set and 4 (20%) patients in the UDCA treated sub-set of patients. Type III ducts (>50 µm) were seen in 4 (11.4%) patients in the DHC treated sub-set and 15 (75%) patients in the UDCA treated sub-set of patients.
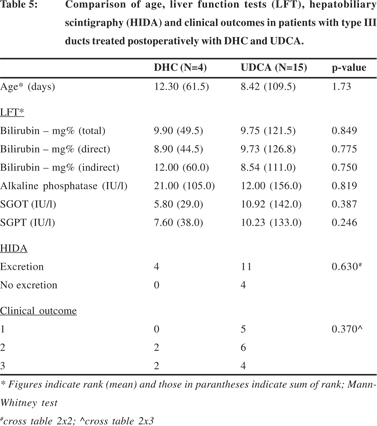
The details of age, liver function tests (LFT), hepatobiliary scintigraphy (HIDA) and clinical outcomes between the types of ducts at the porta hepatis in patients treated postoperatively with DHC and UDCA have been summarized in Tables 1 and 2.
In both the sub-sets the age of the patients had no significant effect on the duct diameter at the porta hepatis (p=0.19 DHC sub-set; p=0.5 UDCA sub-set). Although the post-operative biochemical liver function tests appeared to be better in patients with larger ducts in both sub-sets, the differences were not statistically significant. However, biliary excretion as assessed by hepatobiliary scintigraphy was significantly better in patients with larger ducts in both sub-sets (p=0.003 DHC subset; p=0.025 UDCA sub-set) but this was not reflected in the clinical outcomes of surgery.
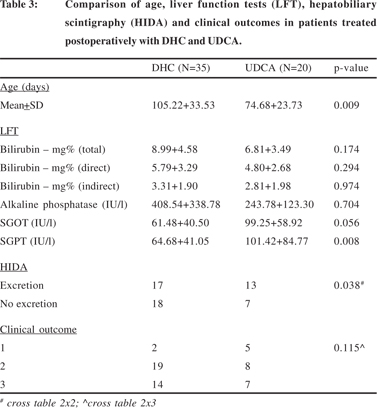
The mean age at operation, means of post-operative biochemical liver function tests, biliary excretion on hepatobiliary scintigraphy and clinical outcomes of surgery between the patients treated post-operatively with DHC and UDCA have been compared in Table 3. The mean age of patients treated with DHC (105.22+33.53 days) was significantly higher than that of the patients treated with UDCA (74.68+23.73) (p=0.009). In the post-operative biochemical liver function tests, the levels of serum bilirubin and serum alkaline phosphatase appeared to be lower in patients treated with UDCA but the differences were not statistically significant. However, in patients treated post-operatively with DHC, the post-operative levels of serum glutamic-oxalacetic transaminase (SGOT) were nearly significantly lower (p=0.056) and those of serum glutamic-pyruvic transaminase (SGPT) were significantly lower (p=0.008). Hepatobiliary scintigraphy showed significantly better biliary excretion in patients treated with UDCA (p=0.003) but this had no effect on the surgical outcome.
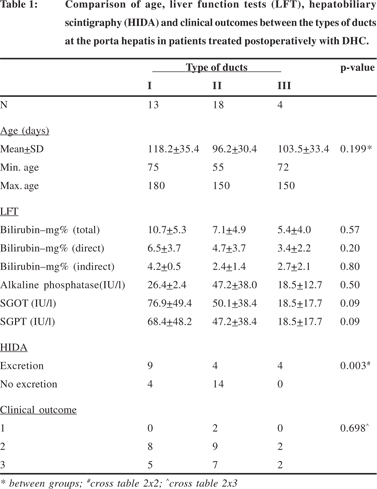
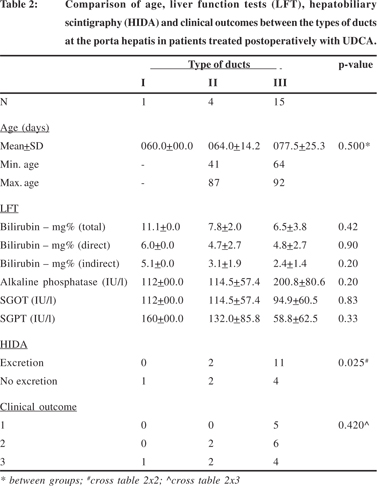
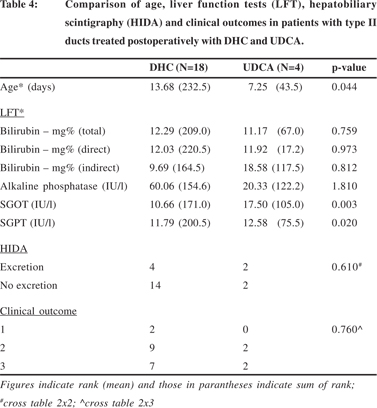
The various parameters i.e. age, liver function tests, biliary excretion on hepatobiliary scintigraphy and surgical outcomes were also compared separately for the type of ducts within the patients treated post-operatively with DHC and UDCA. Since there was only one patient with type I ducts in the sub-set of patients treated with UDCA this comparison was not done for type I ducts. The details for type II and III ducts are summarized in Tables 4 and 5 respectively.
In patients with type II ducts (Table 4), the differences in age, SGOT and SGPT were similar to those described in table 3; the patients were younger in the UDCA sub-set (p=0.044) and the transaminases were lower in the DHC sub-set (p=0.003, SGOT; p=0.02, SGPT). There was no significant difference in the hepatobiliary excretion and surgical outcomes in this subset of patients treated with either DHC or UDCA.
In patients with type III ducts there were no significant differences in age, liver function tests, heptobiliary excretion or surgical outcome (Table 5) between patients treated with either DHC or UDCA.
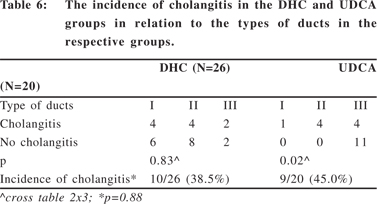
The occurrence and incidence of cholangitis was also studied in these patients (Table 6). In the sub-set of patients treated post-operatively with DHC (n=35), 9 patients died in the early post operative period mainly due to sepsis related complications and hence cholangitis was not documented. In the remaining 26 patients, 10 had documented cholangitis (38.4%) in the first six months after surgery. In the sub-set of patients treated post-operatively with UDCA (n=20), there were no early post-operative deaths and 9/20 (45%) patients had documented cholangitis in the first six months postoperatively.
There was no significant difference in the incidence of cholangitis in the two sub-sets of patients (p=0.88). In the subset of patients treated with DHC the type of ducts did not affect the occurrence of cholangitis but in those treated with UDCA cholangitis was more often seen in patients with no or smaller ducts (p=0.02).
Jaundice free survival was seen in only 2/35 (5.7%) in the DHC sub-set at 5 year follow up; in both these patients the ducts were less than 50µm. In the UDCA subset 5/20 (25%) are alive and jaundice free at 5 years and in all of them the duct size was more than 50µm.
Discussion
The major consideration after hepatic portoenterostomy for EHBA is to maintain a steady flow of bile across the anastomosis. Standard texts of pediatric surgery mention a number of drugs, including bile salts and steroids, that have been tried with variable effects; DHC was administered as part of the post-operative protocol earlier and currently, UDCA has taken the place of DHC.[8,9] Both DHC and UDCA have been shown to have a hepatoprotective and choleretic action and this forms the basis of their use in the post-operative management of EHBA.[10,11]
This study looks at data from two time periods. In the earlier time period (1979-1986) patients with EHBA were administered DHC following the portoenterostomy while in the later period (1999-2002) UDCA was administered. Although these two sub-sets of patients are not strictly comparable, a look at the differences between the two was found to be sufficiently interesting to form the basis of this study, particularly because there is scant data on this aspect.
There was no statistically significant difference in the age at surgery among the three types, categorised according to the duct size, in both DHC and UDCA treated sub-sets, although the patients in the UDCA group were significantly younger as compared to patients in the DHC group. This suggests that in recent times the diagnosis and operation has been done at a younger age as compared to the earlier period. It has been suggested that age at surgery is inversely related to the outcome.[12,13] In the present study, the difference in age of the patients at surgery with the three types of ducts within each sub-set surgery was statistically not significant and hence it was possible to compare the outcome and postoperative LFT results with duct size without the confounding effect of age in each subset. In an earlier study also, although larger ducts were seen in younger patients, we did not find an inverse relationship between age and surgical outcome.[7]
There was no significant difference among the types of ducts within the DHC as well as UDCA sub-sets independently with post-operative biochemical liver function tests. However, when the DHC sub-set was compared with the UDCA sub-set, both the transaminases, SGOT and SGPT, were significantly lower in the DHC sub-set. This difference was not observed with type III ducts but was seen clearly with type II ducts. This may indicate a better hepatoprotective role for DHC as compared with UDCA and this may assume greater importance if the duct size at the porta hepatis is small.
Thirteen of 20 patients in the UDCA sub-set showed excretion into the gut in the postoperative HIDA scan; in these patients the ducts were significantly larger (p=0.025). In the DHC sub-set, 17 of 35 patients had good excretion into the gut and this was also statistically significant (p=0.003). In the present study more patients with ducts larger than 50µm had shown good excretion. These results are in agreement with other series in the literature.[14] However, patients with ducts smaller than 50µm have also shown biliary excretion into the gut and this has also been reported before.[7] In view of the persistently elevated serum bilirubin in the post-operative period, this suggests that although ductal size may have a definite bearing on postoperative bile flow it may still not have any effect on the LFT following Kasai’s portoenterostomy. A search for other factors is required to explain this phenomenon.
Comparing the biliary excretion between the patients in the DHC and UDCA sub-sets, this was seen in a significantly higher number of patients in the UDCA sub-set (p=0.038). Even taking the other factors into consideration, this may suggest that UDCA acts as a better choleretic than DHC to enhance post-operative bile flow. In both the UDCA and DHC sub-sets, cholangitis was seen more often in patients with smaller ducts. ince all the patients had received choleretics, either in the form of DHC or UDCA, it is possible that increase in bile production and flow in the presence of residual obstruction may result in a clinical picture of cholangitis.
Jaundice free survival was seen in only 2/35 (5.7%) in the DHC sub-set at 5 year follow up; in both these patients the ducts were less than 50µm. In the UDCA subset 5/20 (25%) are alive and jaundice free at 5 years and in all of them the duct size was more than 50µm. Thus, factors other than duct size achieve great importance in determining the outcome of surgery e.g. degree of hepatic damage. And, it is obvious that if choleretics have to be of any use then there must be ducts to facilitate the flow of bile. Although bile flow on HIDA is documented in patients without ducts at the porta, long-term survival may be possible with the use of choleretics only if ducts are present.
In conclusion, following Kasai’s portoenterostomy, there was no correlation between the duct size at the porta hepatis with the results of biochemical LFT in patients with EHBA. Patients having larger ducts (>50µm) were associated with good biliary excretion in the postoperative period. This suggests that duct size may have a bearing on postoperative bile flow although this may not be reflected on postoperative LFT. This highlights that LFT may also be dependent on other factors e.g. hepatic parenchymal damage. Cholangitis was seen more often in patients with smaller ducts in both DHC and UDCA sub-sets. This suggests that residual obstruction may be a factor in the causation of clinical cholangitis. The postoperative administration of UDCA seems to be of benefit in patients with ducts larger than 50µm in increasing bile flow and reducing the incidence of cholangitis.
References
1. Kasai M, Ohi R, Chiba T. Intrahepatic bile ducts in biliary atresia. In, Kasai M, Shiraki K, editors. Cholestasis in infancy. Baltimore: University Park Press; 1980. p.181–8.
2. Ohi R, Shikes RH, Stellin GP, Lilly JR. In biliary atresia duct histology correlates with bile flow. J Pediatr Surg. 1984;19:467–70.
3. Kasai M, Suzuki S. A new operation for ‘non-correctable’ biliary atresia : hepatic portoenterostomy. Shujitsu. 1959;13:733–9.
4. Karrer FM, Lilly JR. Corticosteroid therapy in biliary atresia. J Pediatr Surg. 1985;20: 693–5.
5. Vajro P, Couturier M, Lemonnier F, Odievre M. Effects of postoperative cholestyramine and phenobarbital administration on bile flow restoration in infants with extra hepatic biliary atresia. J Pediatr Surg. 1986;21:362–5.
6. Jacquemin E, Hermans D, Myara A, Habes D, Debray D, Hadchouel M, et al. Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis. Hepatology. 1997;25:519–23.
7. Bhatnagar V, Singh MK, Mitra DK. Bile duct diameter at the porta hepatis and liver biopsies in Indian children with biliary atresia. Pediatr Surg Int. 1994;9:558–60.
8. Ohi R, Masaki N. The jaundiced infant : biliary atresia and other obstructions. In, O’Neill JA Jr, Rowe MI, Grosfeld JL, Fonkalsrud EW, Coran AG, editors. Pediatric Surgery. St Louis: Mosby; 1998.p.1465–81.
9. Altman RP, Buchmiller TL. The jaundiced infant : biliary atresia. In, Grosfeld JL, O’Neill JA Jr, Fonkalsrud EW, Coran AG, editors. Pediatric Surgery.St. Louis: Mosby; 2006, p.1603–19.
10. Ratan J, Rohatgi S, Gupta DK, Ratan S. A controlled trial of choleretic and hepatoprotective actions of Livzon and dehydrocholic acid in a model of obstructive jaundice in albino rats. Tohoku J Exp Med.1997;181:161–6.
11. Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–31.
12. Mieli-Vergani G, Howard ER, Portman B, Mowat AP. Late referral for biliary atresia—missed opportunities for effective surgery. Lancet. 1989;1:421–3.
13. Ohi R. Biliary atresia: long term outcomes. In, Howard ER, Stringer MD, Colombani PM, editors. Surgery of the Liver, Bile Ducts and Pancreas in Children. London: Arnold; 2002. p.133–47.
14. Ohi R, Shikes RH, Stellin GP, Lilly JR. In biliary atresia duct histology correlates with bile flow. J Pediatr Surg. 1984;19:467–70.