|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
Hepatitis C, peritransplant management, liver transplantation |
|
|
Manav Wadhawan1, Mukul Rastogi1, Subash Gupta2, Ajay Kumar1
*Dept of Gastroenterology & Hepatology1
*Dept of Surgical Gastroenterology & Liver Transplantation2,
Indraprastha Apollo Hospital,
New Delhi, India.
Corresponding Author:
Dr. Ajay kumar
Email: ajaykge@hotmail.com
DOI:
http://dx.doi.org/
Abstract
Hepatitis C infection is the commonest cause of cirrhosis worldwide. Management of chronic hepatitis C in peritransplant period is challenging. Patients with compensated/ Child class A cirrhosis due to HCV infection are treated like non-cirrhotics, with PEG IFN and Ribavirin, albeit with a higher incidence of complications. Treatment is not recommended for decompensated cirrhotics due to higher complication rate including risk of death. After liver transplant, immunosuppression should be adjusted to prevent/delay recurrent HCV disease. Incidence and severity of recurrent HCV disease as well as patient and graft survival is similar between living donor and deceased donor liver transplants. It is currently recommended to treat established recurrent hepatitis C i.e. raised ALT with HAI>4 and/or F>1. Pre-emptive/prophylactic antiviral therapy is poorly tolerated and has low efficacy. Standard dose regimen (PEG IFN 1.5 mcg/kg or 180 mcg weekly + Ribavirin 800-1200 mg/ day) for 48 weeks irrespective of genotype is the recommended treatment protocol. Therapy poses significant problems in the form of anemia, neutropenia, and a higher risk of rejection.
|
48uep6bbphidcol4|ID 48uep6bbphidvals|302 48uep6bbph|2000F98CTab_Articles|Fulltext Hepatitis C accounts for almost one third of cases of chronic liver disease and is a major cause of liver disease related deaths and causes hepatocellular carcinoma. It represents the most common indication for liver transplantation in India and abroad. Treatment of chronic hepatitis C has been well documented with published guidelines. However, peritransplant management of hepatitis C is a difficult problem and ridden with controversies. In this article we have tried to summarize the Indian data on demography of HCV infection, the problems and results of treatment of HCV infection in compensated and decompensated cirrhosis and also reviewed the current management of HCV recurrence following liver transplantation.
Chronic hepatitis C in India
The community prevalence of hepatitis C in India is 0.4 to 1.5%.[1,2,3] Chronic Hepatitis C is responsible for 15-35% of all chronic liver diseases in India.[4,5,6] The most common genotype in India is 3 (50-80%). Studies from some parts of southern India have a higher prevalence of genotype 1 infection, whereas in northern India genotype 3 is most common.[7,8,9] Virological response of hepatitis C in India varies according to type of IFN used (PEG Interferon {IFN} vs. Standard {Std} IFN), schedule of standard IFN (daily vs. thrice a week {TIW}), genotype (1/4 vs. 2/3), extent of liver disease (chronic hepatitis vs. cirrhosis), severity of cirrhosis (compensated vs. decompensated cirrhosis). The synopsis of available published Indian data is as follows:
1. Sustained virological response (SVR) rates are better with Peg IFN (50%-95%) compared to Std IFN (33%-90%).[10,11]
2. SVR is better with daily IFN (60-90%) compared to TIW IFN (33-75%).[12,13]
3. Genotype 2/3 (50-100%) have a better SVR rates than Genotype 1/4 (33-78%).[10,11,12,13]
4. Non-cirrhotics (50-95%) have a higher SVR than cirrhotics (25-90%).[14,15,16]
5. Higher SVR for compensated cirrhosis (53%) compared to decompensated cirrhosis (32%-46%).[14,15,16]
6. Studies from north India have shown a higher response rate for both genotype 1/4 as well as genotype 2/3 in contrast to studies published from southern India.
7. There is no published data on results of treatment of hepatitis C post liver transplant from India.
Current status of hepatitis C treatment in cirrhosis
In cirrhosis, the rationale for treatment is based on four principles. Treatment may halt progression of disease in a proportion of cases, may prevent the development of HCC in HCV cirrhotics, may decrease the need for transplant in those who respond to treatment and finally it may prevent recurrence of HCV infection post transplant. Treatment of HCV in compensated (Child class A and early Child class B) cirhotics is based on the first three principles. Whereas in decompensated cirrhosis, prevention of disease recurrence after liver transplantation is clearly the main target.
Treatment of hepatitis C in compensated cirrhosis should be identical to that in non-cirrhotic patients (PEG IFN + Ribavirin). There is good evidence that lower dose of PEG IFN _2b (1 mcg/Kg) may be as effective as the standard dose (1.5 mcg/kg).[10,11,14] The incidence of complications during treatment is higher in the cirrhotic patients compared to noncirrhotic population, requiring higher rates dose adjustments and discontinuation. Also there is a risk of decompensation while on treatment which has to be explained to the patient before starting treatment. Dose adjustments have been also quantified according to the level of cytopenias (Table 1).[17]
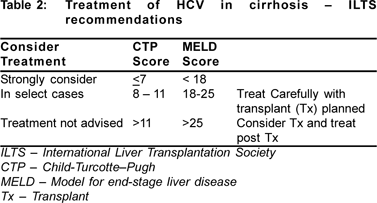
The treatment in decompensated cirrhosis carries a lot of risks. In addition to lower SVRs, treatment causes cytopenias (neutropenia and thrombocytopenia), hemolysis and increase in life-threatening infections specially when treating Child class C cirrhotics. Some subjects may suffer from severe depression and suicidal tendencies, others may have worsening of Child-Pugh score. Frequent dose adjustments (60-100%) are required when treating these patients, a significant proportion are not able to complete treatment due to these problems. Currently International Liver Transplantation Society (ILTS) recommends treatment of hepatitis C in cirrhosis as follows (Table 2).[18]

Role of viral load reduction: Pre transplant setting
Very few trials have studied the efficacy of treatment of HCV with interferon based regimens in patients awaiting liver transplant.[19,20,21,58,59] Five trials published as full papers are summarized in Table 3. Of these studies, four have used Interferon with or without ribavirin, only one trial is on Peg- IFN with ribavirin. The median duration of treatment in these trials vary from 2 – 14 months. The sustained viral response rate at 6 months after transplant in these trials varies between 20-26%. The factors associated with good response to therapy were non-1 genotype, low pretreatment viral load and more than 2 log reduction in viral load at 4 weeks.[19,59] A short, calibrated treatment course for patients awaiting transplant may be specially relevant in a living donor liver transplant program where timing of transplant can be optimally timed.
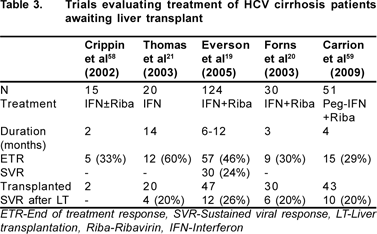
So, from this data it can be inferred that treatment of hepatitis C in patients listed for transplant may decrease recurrence of HCV post transplant in those who respond to treatment. In non-responders, treatment may actually worsen the disease severity.[21,22,59] Considering the poor tolerability and low success rate of HCV treatment post transplant, treatment for hepatitis C may be carefully considered before transplant in a subset of patients listed for transplant. If it is decided to treat patients with decompensated cirrhosis, patient should be listed for transplant before starting treatment. The treatment should be started as per Low Accelerating Dose Regimen (LADR).[19] Dose should be slowly increased to maximum tolerated to achieve best response.
Keypoints
1. All patients with compensated cirrhosis should receive full dose treatment for HCV.
2. Treatment may be considered for cirrhotics with Child score 8-11, with prior listing for transplant
3. Treatment with Child score 8-11 is worthwhile only if SVR expected, (Geno 2/3, low pretransplant viral load, 2 log reduction at week 4)
4. Tolerability is poor with increased incidence of severe adverse events
5. Treatment slows disease progression and improves survival in responders
6. Child score worsens in non responders
7. Treatment is not advised in Child score > 11.
Immunosuppression protocols in HCV related transplants
HCV recurrence is accelerated in transplant recipients due to immunosuppression. Serological recurrence is seen in all patients who are HCV viremic at the time of liver transplant. The type and intensity of immunosuppression is a predictor of severity of recurrence of hepatitis C post transplant. Immunosuppression protocols should be different for HCV recipients as compared to non HCV transplant recipients.
Corticosteroids and Liver Transplant
Corticosteroids enhance HCV replication and may be associated with more severe recurrence of hepatitis C. Steroid boluses cause a marked but transient increase in HCV viremia.[25] However, role of pulse steroids for rejection in increasing HCV recurrence is controversial (Trials before 2000 showing higher incidence, most trials after 2000 showing similar recurrence rates).
Steroid Taper, Withdrawal and Avoidance
Based on role of steroids in HCV replication has prompted multiple trials on steroid withdrawal. Many groups advocate early steroid withdrawal.[60,61] But a number of trials have shown that early taper of steroids (before 6-12 months) should be avoided as it increases severity of HCV recurrence.[23,24]
The jury is still out on the role of complete steroid avoidance in HCV related liver transplants. First published trial is an interim analysis of a study in 39 patients recruited out of a planned total of 50 patients.[62] All patients received tacrolimus and mycomenofolate mofetil (MMF) and were randomized to receive either daclizumab or corticosteroids. There was a trend towards less cellular rejection with daclizumab. However, there was no difference in HCV recurrence other than that the small number of cases with advanced fibrosis all occurred in the corticosteroid arm of the trial. Another prospective trial published recently enrolled 28 patients, used steroid-free immunosuppression (calcineurin inhibitor [CNI], MMF and anti-CD25 antibody) in 18 patients versus steroid-based immunosuppression in 10 patients.[63] Steroid-free immunosuppression was associated with lower incidence of cytomegalovirus (CMV) infection (p = 0.049). Acute cellular rejection rates were similar in both groups. Steroid free group showed a rapid increase in HCV RNA (p < 0.05), and a higher HCV recurrence (46% vs 18.1% at 1 yr, p = 0.009). Currently most centers limit the use of steroids by using initial small doses and withdrawing completely by 6-12 months posttransplant.
Tacrolimus vs Cyclosporin
Cyclosporine has in vitro inhibitory activity on replication of HCV. But in vivo effect has been questioned as most studies show no difference in severity of HCV recurrence with cyclosporin A or tacrolimus.[26,27,28] However, what is documented is that if a patient needs treatment for HCV recurrence with interferon, SVR is higher in those on cyclosporine as primary immunosuppressant compared to tacrolimus.[29] An ongoing multicenter trial is evaluating use of cyclosporine A vs tacrolimus in HCV positive transplant recipients.
Other issues
OKT3 induction or its use for treatment of rejection is associated with increased severity of HCV recurrence.[24] Most centers all over the world have replaced azathioprine (AZA) with MMF in triple immunosuppression. However, in liver transplant the superiority of MMF over AZA is not clearly demonstrated. A very recently published paper has reviewed all trials regarding the use of azathioprine vis a vis MMF in liver transplant patients.[64] These authors report no difference in graft or patient survival between liver transplant recipients treated with MMF or azathioprine. Another recently published trial comparing tacrolimus monotherapy with triple therapy with tacrolimus, azathioprine, and steroids showed a slower onset of histologically proven severe fibrosis and portal hypertension in triple therapy compared to tacrolimus alone.[66] Use of azathioprine has been associated with decreased severity of recurrence of HCV post transplant in other trial also[30,64] and it may be considered along with CNI’s as a part of immunosuppression imen. Rate and severity of HCV recurrence is similar in IL-2 receptor antagonist immunosuppressive regimens compared to conventional regimen.[30] There is no available literature on use of sirolimus in liver transplant for HCV related disease.
Natural history of chronic hepatitis C following liver transplantation
Cirrhosis due to HCV is major indication for OLT in India and in the West accounting for almost 30% of total patients. Graft HCV re-infection is early and universal in patients who are viremic at the time of transplant. 5-yr survival after HCV recurrence in deceased donor liver transplant (DDLT) is lower than non-HCV related liver transplants. Histologic recurrence is seen 50% of these patients at 1 yr post transplant. Cumulative probability of graft cirrhosis is 30% at 5 years post transplant, as compared to 5% in chronic HCV infection in non transplanted population. Actuarial risk of decompensation once these transplant recipients develop cirrhosis is 42% at 1 yr, 62% at 5 yrs; whereas in non transplant HCV it is 5% and 10% at 1 and 3 yrs respectively. 3-yr survival after decompensation is less than 10% for recurrent HCV after liver transplantation. Of total number of patients transplanted for HCV related liver disease, 10-25% will need retransplantation within 5 years. All this data is from cadaveric liver transplants.[31,32,33]
Initial comparative data of severity of HCV recurrence following living donor liver transplant (LDLT) vs. Deceased donor liver transplant (DDLT) showed greater severity of recurrence and poorer 1 yr graft survival.[34,35] Subsequently, many trials have been published which show no difference in mean inflammation score or mean fibrosis score, with similar occurrence of cirrhosis and similar graft and patient survival at 4 yrs, in patients with recurrent HCV in LDLT vs. DDLT. [33,36,37,38,67].
Keypoints
1. Majority of studies points toward no differences in 1- and 5-yr graft and patient survival between LDLT and DDLT
2. No differences in 1- to 3-yr inflammation scores, fibrosis and cirrhosis.
Do all patients with HCV need treatment post liver transplant?
Treatment protocols for HCV post liver transplant can be broadly divided in 2 main categories: treatment for all irrespective of ALT and liver biopsy findings (pre-emptive therapy) and treatment of recurrent disease (ALT raised, liver biopsy HAI>4 and/or Fibrosis >1). Multiple trials of pre-emptive therapy have shown a pooled end of treatment response (ETR) of 13.6% and a sustained viral response (SVR) of 9.1%.[39] The incidence of complications has been 60-90% (dose reduction - 85%, discontinuation - 37%, serious adverse events - 27%). Only 15% of total patients were able to receive complete and full dose treatment. However, one recent trial published from Japan has shown a 45% ETR and 39% SVR after preemptive treatment which continued for 1yrafter 1st negative HCV RNA report.[65] This study also showed similar survival between patients transplanted for HCV and non HCV cirrhosis.[65]
The minimum criteria for established disease needing treatment are raised ALT levels, HAI>4 and/or Fibrosis >1 on liver biopsy score according to modified Ishak’s system.[18] The pooled SVR rate on IFN+Ribavirin combination (27 published trials) has been reported to be 24% (13% -53%). Pooled discontinuation rate in these studies was 24%. On the combination of PEG-IFN and Ribavirin (21 studies), pooled SVR rate was slightly higher {27%(26%-50%)}, and so was the pooled discontinuation rate (26%).[40] Acute cellular rejection was reported in 2% (0-4%) receiving IFN and 5% (2-25%) of those receiving PEG-IFN.
Keypoints
1. Pre-emptive/prophylactic antiviral therapy is poorly tolerated and has low efficacy
2. It is currently recommended to treat established disease i.e. raised ALT with HAI>4 and/or F>1.
Current treatment protocol of treatment of HCV post Liver Transplant
Treatment is recommended for established HCV recurrence post transplant only (HAI>4, F>1).[18] Two treatment protocols are followed; escalating dose regimen – Peg IFN (0.5-1.5 mcg/kg or 90/135/180 mcg weekly) + Ribavirin (400-1200 mg/day) or standard dose regimen (PEG IFN 1.5 mcg/kg or 180 mcg weekly + Ribavirin 800-1200 mg/day). Most centers prefer the standard dose over escalating regimen. Treatment should be started at fibrosis score 1-2 as response rates decrease with higher fibrosis scores at the start of treatment (48% vs. 19%).[41] The duration of treatment is recommended to be 48 weeks irrespective of the genotype.
Keypoints
1. Standard dose regimen (PEG IFN 1.5 mcg/kg or 180 mcg weekly + Ribavirin 800-1200 mg/day) is the recommended treatment protocol.
2. HAI>4, F>1 should be treated. Delaying treatment till higher fibrosis scores decreases the response rate.
3. 48 weeks treatment is recommended irrespective of HCV genotype.
Post liver transplant problems in HCV treatment
Treatment of HCV recurrence post liver transplant is difficult. Problems can be related to drug discontinuation / dose reduction of Peg IFN and/or Ribavirin, risk of graft rejection or other miscellaneous issues like Post Transplant Diabetes Mellitus (PTDM) CMV, occult HBV, etc.
Anaemia is seen in 36-81% LT recipients treated for chronic hepatitis C.[42] The mechanisms can be multiple: ribavirin induces hemolysis by accumulation of phosphorylated ribavirin in RBCs causing oxidative injury,[43] interferon induced myelosuppression contributing to anemia,[44] HCV interference in erythropoietin production (EPO) production by direct inhibition as well as ribavirin induced renal insufficiency.[45] Treatment is by using weight based dosing of ribavirin, use of erythropoietin(EPO) which is started pre-emptively when hemoglobin (Hb) <10gm% or when there is >2 gm% reduction in Hb. More vigilance is required in the elderly and in those on concurrent MMF.
Neutropenia and thrombocytopenia is reported in up to 50% of patients on treatment with PEG-IFN+Riba. Treatment discontinuation is reported in 0-40% of patients in various studies.[46,47,48,49,50] Usual management is by reducing the dose/ frequency of PEG-IFN and restarting normal dosage once it comes back to normal. Granulocyte macrophage colony stimulating factor (Gm-CSF) is also used concurrently to increase counts while on treatment.
One of the most important risks of treatment for HCV is graft rejection. It has been variously reported between 0-25% in various published studies.[46,47,48,49,50] The proposed mechanism of rejection is that interferon enhances expression of HLADR antigens on donor bile duct epithelial cells and hepatocytes. Further, anti-viral therapy improves hepatocyte microsomal function leading to decreased immunosuppression levels.[42] Median time to rejection has been reported to be 3.5 months (range 0.3-15.7 months) after HCV RNA becomes undetectable.[51] The incidence of rejection is shown to be higher in responders vs. nonresponders. [51] Another study has reported a higher incidence of fibrosing cholestatic hepatitis (FCH) in non-responders after stopping IFN therapy.[52] Risk of chronic rejection is miniscule and overstated.
Post Transplant Diabetes Mellitus (PTDM) is seen in 7.2% of liver transplant recipients at 6 months post transplant. Incidence is higher with tacrolimus than cyclosporine, and 4 to 8 fold higher prevalence is seen in HCV related liver transplants than in non-HCV transplants. Exact mechanism is not known but both HCV direct effect and immune mediated effects have been proposed. It is recommended that insulin be used in thin patients and oral hypoglycemics in overweight patients.[53] Sulfonylureas and thiazolidinediones have been found to be safe.[53,54,55] Metformin is not to be used post liver transplant for the concern of lactic acidosis.
Cytomegalovirus infection does not increase the risk of HCV recurrence post transplant. There is no difference in HCV viral load, degree of liver injury and fibrosis in CMV PCR positive versus negative patients with HCV related liver transplant.[56] On the other hand CMV viral load is similar between HCV related and non HCV liver transplants.[56]
Keypoints
1. Anemia is seen in seen in a majority of patients on PEG + Riba treatment post transplant. Treatment is by erythropoietin.
2. Neutropenia is reversible by decreasing PEG-IFN dose. Gm-CSF is also used for increasing neutrophil counts.
3. Acute graft rejection is seen in 0-25% patients and mainly occurs in responders to anti-viral therapy
4. There is a risk of FCH on discontinuing anti-viral treatment in non-responders.
5. PTDM is more common in HCV related liver transplants. Insulin and oral hypoglycemics (sulfonylureas and thiazolidinediones) are safe in post transplant setting.
6. CMV status has no significant effect on HCV recurrence.
Conclusion
Hepatitis C infection accounts for maximum end stage liver disease patients. Management of chronic hepatitis C in peritransplant period requires specialized care by dedicated hepatologists. Patients with compensated/ Child class A cirrhosis due to HCV infection should be treated like chronic hepatits C patients without cirrhosis. The risk of treatment associated complications is much higher in this group. Decompensated cirrhotics have a very high risk complications including risk of further worsening of liver disease and death. After liver transplant, immunosuppression should be adjusted to prevent/delay recurrent HCV disease. There is no difference in the incidence and severity of recurrent HCV disease as well as patient and graft survival between living donor and deceased donor liver transplants. It is currently recommended to treat established recurrent hepatitis C post liver transplant. The minimum criteria for treatment are raised ALT with HAI>4 and/or F>1 on liver biopsy. Pre-emptive/prophylactic antiviral therapy is poorly tolerated and has low efficacy. Standard dose regimen (PEG-IFN 1.5 mcg/kg or 180 mcg weekly + Ribavirin 800-1200 mg/day) for 48 weeks irrespective of genotype is the recommended treatment protocol. Therapy is associated with a high complication rate in the form of anemia, neutropenias, higher risk of rejection and fibrosing cholestatic hepatitis.
References
1. Mishra S, Chayani N, Sarangi G, Mallick B, Pati SB. Seroprevalence of anti HCV antibody in and around Cuttack, Orissa. Indian J Med Microbiol. 2002;20:40–1.
2. Bhatia R. Blood transfusion services in developing countries of South-East Asia. Transfus Today. 2005;65:4–5.
3. Kapoor D, Saxena R, Sood B, Sarin SK. Blood transfusion practices in India: results of a national survey. Indian J Gastroenterol. 2000;19:64–7.
4. Issar SK, Ramakrishna BS, Ramakrishna B, Christopher S, Samuel BU, John TJ. Prevalence and presentation of hepatitis C related chronic liver disease in southern India. J Trop Med Hyg. 1995;98:161–5.
5. Berry N, Chakravarti A, Das U, Kar P, Das BC, Mathur MD. HCV seroreactivity and detection of HCV RNA in cirrhotics. Diagn Microbiol Infect Dis. 1999;35:209–13.
6. Ray G, Ghoshal UC, Banerjee PK, Pal BB, Dhar K, Pal AK, Biswas PK. Aetiological spectrum of chronic liver disease in eastern India. Trop Gastroenterol. 2000;21:60–2.
7. Hissar SS, Goyal A, Kumar M, Pandey C, Suneetha PV, Sood A, et al. Hepatitis C virus genotype 3 predominates in North and Central India and is associated with significant histopathologic liver disease. J Med Virol. 2006;78:452–8.
8. Chandra M, Thippavuzzula R, Ramachandra Rao VV, Habib AM, Habibullah CM, Narasu L, et al. Genotyping of Hepatitis C virus (HCV) in infected patients from South India. Infect Genet Evol. 2007;7:724–30.
9. Amarapurkar D, Dhorda M, Kirpalani A, Amarapurkar A, Kankonkar S. Prevalence of hepatitis C genotypes in Indian patients and their clinical significance. J Assoc Physicians India. 2001;49:983–5.
10. Sood A, Midha V, Hissar S, Kumar M, Suneetha PV, Bansal M, et al. Comparison of low-dose pegylated interferon versus standard high-dose pegylated interferon in combination with ribavirin in patients with chronic hepatitis C with genotype 3: an Indian experience. J Gastroenterol Hepatol. 2008;23:203–7.
11. Gupta R, Ramakrishna CH, Lakhtakia S, Tandan M, Banerjee R, Reddy DN. Efficacy of low dose peginterferon alpha-2b with ribavirin on chronic hepatitis C. World J Gastroenterol. 2006;12:5554–6.
12. Hazari S, Panda SK, Gupta SD, Batra Y, Singh R, Acharya SK. Treatment of hepatitis C virus infection in patients of northern India. J Gastroenterol Hepatol. 2004;19:1058–65.
13. Sarin SK, Goyal A, Kumar S, Guptan RC, Hashmi AZ, Sakhuja P, et al. A randomized trial of a 4- vs 12-week daily interferon dose regimen combined with ribavirin in treatment of patients with chronic hepatitis C. Hepatobiliary Pancreat Dis Int. 2004;3:42–8.
14. Sood A, Midha V, Sood N, Bansal M. Pegylated interferon alfa 2b and oral ribavirin in patients with HCV-related cirrhosis. Indian J Gastroenterol. 2006;25:283–5.
15. Amarapurkar DN, Patel ND, Kamani P. Antiviral therapy of decompensated cirrhosis due to hepatitis C viral infection. Trop Gastroenterol. 2005;26:119–22.
16. Kumar R, Kumar S, Sharma BC, Singh J, Sarin SK. Antiviral therapy in advanced chronic liver disease due to hepatitis C virus infection: pilot study. J Gastroenterol Hepatol. 2005;20:527–35.
17. Tekin F, Gunsar F, Karasu Z, Akarca U, Ersoz G. Safety, tolerability, and efficacy of pegylated-interferon alfa-2a plus ribavirin in HCVrelated decompensated cirrhotics. Aliment Pharmacol Ther. 2008;27:1081–5.
18. Wiesner RH, Sorrell M, Villamil F; International Liver Transplantation Society. Expert panel. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9:S1–9.
19. Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255–62.
20. Forns X, Garcia-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389–96.
21. Thomas RM, Bremms JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9:905–15.
22. Iacobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, et al. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206–12.
23. Samonakis DN, Triantos CK, Thalheimer U, Quaglia A, Leandro G, Teixeira R, et al. Immunosuppression and donor age with respect to severity of HCV recurrence after liver transplantation. Liver Transpl. 2005;11:386–95.
24. Berenguer M, Aguilera V, Prieto M, San Juan F, Rayón JM, Benlloch S, Berenguer J. Significant improvement in the outcome of HCVinfected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J Hepatol. 2006;44:717–22.
25. Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:167–77.
26. Martin P, Busuttil RW, Goldstein RM, Crippin JS, Klintmalm GB, Fitzsimmons WE, et al. Impact of tacrolimus versus cyclosporine in hepatitis C virus-infected liver transplant recipients on recurrent hepatitis: a prospective, randomized trial. Liver Transpl. 2004;10:1258–62.
27. Zervos XA, Weppler D, Fragulidis GP, Torres MB, Nery JR, Khan MF, et al. Comparison of tacrolimus with microemulsion cyclosporine as primary immunosuppression in hepatitis C patients after liver transplantation. Transplantation. 1998;65:1044–6.
28. Berenguer M, Aguilera V, Prieto M, San Juan F, Rayón JM, Benlloch S, et al. Effect of calcineurin inhibitors on survival and histologic disease severity in HCV-infected liver transplant recipients. Liver Transpl. 2006;12:762–7.
29. Firpi RJ, Abdelmalek MF, Soldevila-Pico C, Reed A, Hemming A, Howard R, et al. Combination of interferon alfa-2b and ribavirin in liver transplant recipients with histological recurrent hepatitis C. Liver Transpl. 2002;8:1000–6.
30. Walter T, Dumortier J, Guillaud O, Hervieu V, Scoazec JY, Boillot O. Factors influencing the progression of fibrosis in patients with recurrent hepatitis C after liver transplantation under antiviral therapy: a retrospective analysis of 939 liver biopsies in a single center. Liver Transpl. 2007;13:294–301.
31. Berenguer M, Prieto M, Rayón JM, Mora J, Pastor M, Ortiz V, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–8.
32. Charlton M, Ruppert K, Belle SH, Bass N, Schafer D, Wiesner RH,et al. Long-term results and modeling to predict outcomes in recipients with HCV infection: results of the NIDDK liver transplantation database. Liver Transpl. 2004;10:1120–30.
33. Guo L, Orrego M, Rodriguez-Luna H, Balan V, Byrne T, Chopra K, et al. Living donor liver transplantation for hepatitis C-related cirrhosis: no difference in histological recurrence when compared to deceased donor liver transplantation recipients. Liver Transpl. 2006;12:560–5.
34. Gaglio PJ, Malireddy S, Levitt BS, Lapointe-Rudow D, Lefkowitch J, Kinkhabwala M, et al. Increased risk of cholestatic hepatitis C in recipients of grafts from living versus cadaveric liver donors. Liver Transpl. 2003;9:1028–35.
35. Thuluvath PJ, Yoo HY. Graft and patient survival after adult live donor liver transplantation compared to a matched cohort who received a deceased donor transplantation. Liver Transpl. 2004;10:1263–8.
36. Shiffman ML, Stravitz RT, Contos MJ, Mills AS, Sterling RK, LuketicVA, et al. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl. 2004;10:1248–55.
37. Rodriguez-Luna H, Vargas HE, Sharma P, Ortiz J, De Petris G, Balan V, et al. Hepatitis C virus recurrence in living donor liver transplant recipients. Dig Dis Sci. 2004;49:38–41.
38. Bozorgzadeh A, Jain A, Ryan C, Ornt D, Zand M, Mantry P, et al. Impact of hepatitis C viral infection in primary cadaveric liver allograft versus primary living-donor allograft in 100 consecutive liver transplant recipients receiving tacrolimus. Transplantation. 2004;77:1066–70.
39. Shergill AK, Khalili M, Straley S, Bollinger K, Roberts JP, Ascher NA, Terrault NA. Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. Am J Transplant. 2005;5:118–24.
40. Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferonbased combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006;6:1586–99.
41. Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007;132:1746–56.
42. Saab S, Oh MK, Ibrahim AB, Durazo F, Han S, Yersiz H, et al. Anemia in liver transplant recipients undergoing antiviral treatment for recurrent hepatitis C. Liver Transpl. 2007;13:1032–8.
43. De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004.
44. Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in haemoglobin during interferon alpha-2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11:243–50.
45. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–40.
46. Dumortier J, Scoazec JY, Chevallier P, Boillot O. Treatment of recurrent hepatitis C after liver transplantation: a pilot study of peginterferon alfa-2b and ribavirin combination. J Hepatol. 2004;40:669–74.
47. Castells L, Vargas V, Allende H, Bilbao I, Luis Lázaro J, Margarit C, Esteban R, et al. Combined treatment with pegylated interferon (alpha-2b) and ribavirin in the acute phase of hepatitis C virus recurrence after liver transplantation. J Hepatol. 2005;43:53–9.
48. Neumann U, Puhl G, Bahra M, Berg T, Langrehr JM, Neuhaus R, et al. Treatment of patients with recurrent hepatitis C after liver transplantation with peginterferon alfa-2B plus ribavirin. Transplantation. 2006;82:43–7.
49. Berenguer M, Palau A, Fernandez A, Benlloch S, Aguilera V, Prieto M, et al.. Efficacy, predictors of response, and potential risks associated with antiviral therapy in liver transplant recipients with recurrent hepatitis C. Liver Transpl. 2006;12:1067–76.
50. Sharma P, Marrero JA, Fontana RJ, Greenson JK, Conjeevaram H, Su GL, et al. Sustained virologic response to therapy of recurrent hepatitis C after liver transplantation is related to early virologic response and dose adherence. Liver Transpl. 2007;13:1100–8.
51. Kugelmas M, Osgood MJ, Trotter JF, Bak T, Wachs M, Forman L, et al. Hepatitis C virus therapy, hepatocyte drug metabolism, and risk for acute cellular rejection. Liver Transpl. 2003;9:1159–65.
52. Kornberg A, Küpper B, Tannapfel A, Thrum K, Bärthel E, Settmacher U. Antiviral treatment withdrawal in viremic HCVpositive liver transplant patients: impact on viral loads, allograft function and morphology. Liver Int. 2006;26:811–6.
53. Salvadori M, Bertoni E, Rosati A, Zanazzi M. Post-transplant diabetes mellitus. J Nephrol. 2003;16:626–34.
54. Villanueva G, Baldwin D. Rosiglitazone therapy of posttransplant diabetes mellitus. Transplantation. 2005;80:1402–5.
55. Luther P, Baldwin D Jr. Pioglitazone in the management of diabetes mellitus after transplantation. Am J Transplant. 2004;4:2135–8.
56. Nebbia G, Mattes FM, Cholongitas E, Garcia-Diaz A, Samonakis DN, Burroughs AK, et al. Exploring the bidirectional interactions between human cytomegalovirus and hepatitis C virus replication after liver transplantation. Liver Transpl. 2007;13(1):130–5.
57. McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or afa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–93.
58. Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350–5.
59. Carrión JA, Martínez-Bauer E, Crespo G, Ramírez S, Pérez-del- Pulgar S, García-Valdecasas JC, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: A retrospective study. J Hepatol. 2009;50:719–28.
60. Everson GT, Trouillot T, Wachs M, Bak T, Steinberg T, Kam I, et al. Early steroid withdrawal in liver transplantation is safe and beneficial. Liver Transpl Surg. 1999;5:S48–57.
61. Burroughs AK. Posttransplantation prevention and treatment of recurrent hepatitis C. Liver Transpl. 2000;6:S35–40.
62. Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid-free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplant Proc. 2005;37:1217–9.
63. Marubashi S, Dono K, Nagano H, Kim C, Asaoka T, Hama N, et al. Steroid-free living donor liver transplantation in adults: impact on hepatitis C recurrence. Clin Transplant. 2009;23:904–13.
64. Germani G, Pleguezuelo M, Villamil F, Vaghjiani S, Tsochatzis E, Andreana L, et al.. Azathioprine in liver transplantation: a reevaluation of its use and a comparison with mycophenolate mofetil. Am J Transplant. 2009;9:1725–31.
65. Tamura S, Sugawara Y, Yamashiki N, Kaneko J, Kokudo N, Makhuuchi M. Pre-emptive antiviral therapy in living donor liver transplantation for hepatitis C: observation based on a singlecenter experience. Transpl Int. 2009 Dec 15. [Epub ahead of print].
66. Manousou P, Samonakis D, Cholongitas E, Patch D, O’Beirne J, Dhillon AP, et al. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transpl. 2009;15:1783–91.
67. Gallegos-Orozco JF, Yosephy A, Noble B, Aqel BA, Byrne TJ, Carey EJ, et al. Natural history of post–liver transplantation hepatitis C: A review of factors that may influence its course. Liver Transpl.2009;15:1872–81.
|
|
|
 |
|
|