48uep6bbphidvals|255
48uep6bbphidcol4|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
Lymphoproliferative disorders, especially non-Hodgkin’s lymphoma, occur at an increased rate in immunodeficient or immunosuppressed patients.[1] An increased risk of lymphoma has also been reported in patients with Crohn’s disease (CD).[2] However, most cases of lymphoma associated with infliximab; a chimeric IgG1 monoclonal antibody that binds specifically and directly to human tumor necrosis factor-a (TNF-a); were of the hepatosplenic T cell or the B-cell non-Hodgkin’s types.[3,4,5,6]
We present a case of nodular sclerosing Hodgkin’s lymphoma (HL), in a patient receiving long-term azathioprine and infliximab therapy for CD. The possible relationship between this lymphoproliferative neoplasm and concurrent therapy with TNF antagonists is explored.
Case Summary
In May 2004, a 35 year-old Saudi patient was referred to King Faisal Specialist Hospital and Research Center with active colonic CD refractory to oral steroid and sulfasalazine therapy. After clinical, biochemical, radiological and pathological assessments, the diagnosis was confirmed and the patient was started on oral daily doses of prednisone 50 mg, azathioprine 100 mg, mesalamine 3 gram, and his sulfasalazine was stopped. Prednisone treatment was tapered over 5 weeks to 10 mg daily. Three months later, he showed no significant improvement, and started on infliximab (Remicade; Schering-Plough, Holland) induction therapy of three doses of 5 mg/Kg at 0, 2 and 6 weeks after excluding hepatitis B virus, cytomegalovirus (CMV), and mycobacterial tuberculosis infections.
Patient received 18 IV infliximab infusions (each was 300 mg, with IV diphenhydramine 25 mg and hydrocortisone 100 mg pre-medication) from September 2004 till June 2007 with remarkable clinical, biochemical, and endoscopic responses. He had a smooth course, only interrupted by an episode of CMV colitis that was treated effectively with ganciclovir and follow up CMV antigenemia was negative.
In June 2007, he presented with right-sided neck swellings associated with 3 months history of intermittent fever, night sweats, and weight loss of 6 kilograms, without any diarrhoea or abdominal pains. Clinically, he was afebrile, hemodynamically stable with non-tender enlarged rubbery right cervical lymph nodes, the largest was 2x2 cm. Chest, cardiac, and abdominal examinations were unremarkable, and the clinical impression was inactive CD with new lymphadenopathy.
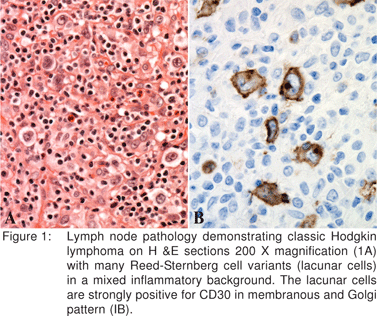
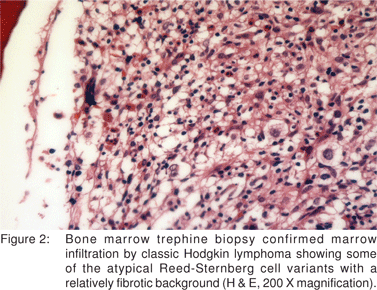
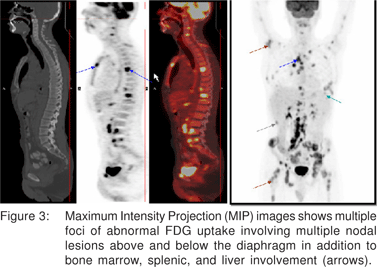
Laboratory investigations were as follows. WBC: 7,800/ mL, hemoglobin: 110 g/L, platelets: 256,000/mL, albumin: 38 g/L, alkaline phosphatase: 239, ESR: 98 mm/hour, CRP: 174 mg/L. Excision lymph node biopsy showed classic nodular sclerosis HL with many Reed-Sternberg cell variants (lacunar cells) in a mixed inflammatory background, strongly positive for CD30 in membranous and Golgi pattern (Figure 1). Bone marrow examination and biopsy confirmed marrow infiltration by classic HL showing some of the atypical Reed-Sternberg cell variants with a relatively fibrotic background (Figure 2). His whole body F18 FDG PET-CT was nodal involvement above and below the diaphragm, together with the bone marrow, spleen, and liver (Figure 3). Infliximab was, then, put on hold, but the patient continued on oral pentasa and azathioprine, and was referred to Oncology.
Two weeks later, and before starting chemotherapy, he presented to the emergency room with septic shock secondary to severe right lower lobe pneumonia, that required Intensive Care Unit admission, intubation, inotropic support, septic screen, and IV omeprazole, hydrocortisone, Piperacillin-tazobactam and clarithromycin therapies, and his prednisone and azathioprine were stopped. CMV tests were negative. Epstein-Barr virus (EBV) serology showed EBV IgA <1:10, early antigen 7.06 AU/mL (positive), EBV IgM <5.00 AU/mL, EBV nuclear antigen 25.11AU/mL (positive), EBV VCA IgG >170.00 AU/mL, and EBV load of 34140 copies/mL. Bronchoscopy showed thick yellowish sputum in right lower lobe bronchial tree, and bronchoalveolar lavage was negative for acid fast bacilli, gram stain and culture, CMV, and fungal studies, but his blood culture grew E. coli. Patient made full recovery and was discharged home with follow up with the Oncology clinic. He received 8 cycles of adriamycin, bleomycin (initially avoided in first two cycles due to active pulmonary infection), vincristine and prednisone (ABVD) with initial shrinkage of his lymph nodes. However, spine MRI revealed multiple bone metastates without cord compression. Therefore, salvage chemotherapy with etoposide, methylprednisolone, cytarabine, and cisplatin was initiated. Unfortunately, he developed another severe refractory septic shock with multi-organ failure after the second cycle, and passed away in November 2008 despite intensive therapy.
Discussion
This case of extra-intestinal nodular-sclerosing HL in a patient on long-term maintenance therapy with infliximab and azathioprine for CD is one of the very few reported in literature. Infliximab associated lymphomas are mainly of hepatosplenic T-cell or the B-cell non-Hodgkin’s types.[3,4,5,6] Chances of lymphoma development irrespective of the underlying disease increase if infliximab is used with another immunosuppressive agent as ciclosporin and methotrexate.[5,6], This patient was also on azathioprine, and indeed, a multi-center study did not show any significant ncrease in the incidence of neoplasia in patients with CD treated with infliximab when compared with those who did not receive it.[7]
IBD may be associated with development of lymphoma. This association was totally rejected in a report,[8] but it was raised in others.[9,10] Although reports implicate ulcerative colitis more than CD in this issue, other reports show that CD can rarely be associated with extra-intestinal HL, even without immunosuppressive therapy.[11,12] By looking at these reports and at our case, CD itself can be implicated as a potential predisposing factor in the pathogenesis of HL in our patient, putting in mind its occurrence in the extraintestinal organs.
Another important feature of immunosuppressionassociated lymphomas is the well-established relationship between many of these malignancies and EBV infection.[13,14,15] However, in this patient evidence of infection with this virus was positive only in blood serology, but absent in histopathology specimens of both bone marrow and lymph node biopsies. Moreover, EBV-associated HL usually involves the colorectal region, [13,14,15] and the small bowel, but our patient’s HL was extra-intestinal.
There is a strong association between CMV infection and Non-Hodgkin’s lymphoma.[16] Indeed, our patient had CMV colitis that was treated successfully and a repeat test for CMV antigenemia was negative at the time he presented with HL, making CMV a less likely cause. Moreover, CMV infection mostly occurs as a complication of lymphoma or its radio- or chemotherapy rather than as an etiology. In addition, we could not find any evidence of association between HL and CMV infection.
Azathioprine is used to maintain remission, and as a steroid-sparing agent in patients with CD. Long-term use of azathioprine has previously been implicated in the pathogenesis of reversible lymphoma in patients with IBD.[17,18] However, its benefits clearly outweighs the risk of lymphoma,[19] and justifies its use in our case, and in others. Whether this risk increases with concomitant and prolonged use of infliximab needs to be evaluated.
In conclusion, infliximab alone can not be held responsible for the pathogenesis of HL in this patient as he had two other risk factors namely his primary disease and the use of azathioprine, but both higher index of suspicion and closer follow up are required if patients are maintained on long-term infliximab together with other immunosuppressive therapy. Both physicians and patients need to be aware of this potential rare complication.
References
1. Beral V, Newton R. Overview of the epidemiology of immunodeficiency-associated cancers. J Natl Cancer Inst Monogr. 1998;23:1–6.
2. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62.
3. Nakashima C, Tanioka M, Takahashi K, Miyachi Y. Diffuse large B-cell lymphoma in a patient with rheumatoid arthritis treated with infliximab and methotrexate. Clin Exp Dermatol. 2008;33:437–9.
4. ZeidanA, Sham R, Shapiro J, BarattaA, Kouides P. Hepatosplenic T-cell lymphoma in a patient with Crohn’s disease who received infliximab therapy. Leuk Lymphoma. 2007;48:1410–3.
5. Bucher C, Degen L, Dirnhofer S, Pless M, Herrmann R, Schraml P, et al. Biologics in inflammatory disease: infliximab associated risk of lymphoma development. Gut. 2005;54:732–3.
6. Mahé E, Descamps V, Grossin M, Fraitag S, Crickx B. CD30+ Tcell lymphoma in a patient with psoriasis treated with ciclosporin and infliximab. Br J Dermatol. 2003;149:170–3.
7. Biancone L, Orlando A, Kohn A, Colombo E, Sostegni R, Angelucci E, et al. Infliximab and newly diagnosed neoplasia in Crohn’s disease: a multicentre matched pair study. Gut. 2006;55:228–33.
8. Lewis JD, Bilker WB, Brensinger C, Deren JJ, Vaughn DJ, Strom BL. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121:1080–7.
9. Palli D, Trallori G, Bagnoli S, Saieva C, Tarantino O, Ceroti M, et al. Hodgkin’s disease risk is increased in patients with ulcerative colitis. Gastroenterology. 2000;119:647–53.
10. Loftus EV Jr, Sandborn WJ. Lymphoma risk in inflammatory bowel disease: influences of referral bias and therapy. Gastroenterology. 2001;121:1239–42.
11. Musso M, Porretto F, Crescimanno A, Bondì F, Polizzi V, Scalone R. Crohn’s disease complicated by relapsed extranodal Hodgkin’s lymphoma: prolonged complete remission after unmanipulated PBPC autotransplant. Bone Marrow Transplant. 2000;26:921–3.
12. Calvo-Villas JM, Ramirez Sanchez MJ, Cuesta Tovar J, García C. Extraintestinal Hodgkin’s disease in a patient with Crohn’s disease. South Med J. 2003;96:632.
13. Wong NA, Herbst H, Herrmann K, Kirchner T, Krajewski AS, Moorghen M, et al. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: detection of the virus in lymphomas but not in adenocarcinomas. J Pathol. 2003;201:312–8.
14. Kumar S, Fend F, Quintanilla-Martinez L, Kingma DW, Sorbara L, Raffeld M, et al. Epstein-Barr virus-positive primary gastrointestinal Hodgkin’s disease: association with inflammatory bowel disease and immunosuppression. Am J Surg Pathol. 2000;24:66–73.
15. Bai M, Katsanos KH, Economou M, Kamina S, Balli C, Briasoulis E, et al. Rectal Epstein-Barr virus-positive Hodgkin’s lymphoma in a patient with Crohn’s disease: case report and review of the literature. Scand J Gastroenterol. 2006;41:866–9.
16. Daniel F, Damotte D, Moindrot H, Molina T, Berger A, Cellier C. A steroid-refractory ulcerative colitis revealing Epstein-Barr virus/ cytomegalovirus-positive colonic lymphoma. Int J Colorectal Dis. 2006,21:288–90.
17. Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet . 1994,343:1249–52.
18. Larvol L, Soule JC, Le Tourneau A. Reversible lymphoma in the setting of azathioprine therapy for Crohn’s disease. N Engl J Med. 1994,331:883–4.
19. Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000,118:1018–24.