|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
hepatocellular carcinoma, transarterial chemoembolisation, oral chemotherapy and targeted molecular therapy |
|
|
Shashi B Paul,1 YC Manjunatha1, Subrat K Acharya2
Departments of Radiodiagnosis1 and Gastroenterology2
All India Institute of Medical Sciences
New Delhi, India.
Corresponding Author:
Prof. Subrat Kumar Acharya
Email: subratacharya2004@yahoo.com
DOI:
http://dx.doi.org/
Abstract
Hepatocellular carcinoma (HCC) is a global health problem, the fifth most common cancer in the world. HCC occurs in a histologically abnormal liver due to underlying chronic liver disease resulting as a sequele of the chronic viral infections, hepatitis B and C. Since these two viral infections are endemic in Asia and Africa , more than 80% of cases are encountered in these regions. In , a large proportion of the population is “at risk” for developing chronic liver disease and, therefore, HCC. Due to the lack of screening programmes in the country, the majority of HCC patients are diagnosed at an advanced stage of the disease, and thus treatment remains a challenge. Palliative therapy forms the mainstay of treatment for this group of patients. The current era provides a plethora of options for the palliative management of HCC. This review concisely summarises the historical perspective and the current status of palliative treatment in advanced HCC.
|
48uep6bbphidvals|262 48uep6bbphidcol4|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Hepatocellular carcinoma (HCC) is a global health problem and the fifth most common cancer.[1] In approximately 80% of cases, HCC occurs in a histologically abnormal liver due to underlying chronic liver disease resulting from the chronic viral infections, hepatitis B or C. Since these two viral infections are endemic in Asia and Africa, more than 80% of cases are encountered in these regions.[2] HCC forms 5.7% of new cancer cases diagnosed each year, and of these, 82% cases and deaths occur in developing countries.[3] India lies in the intermediate endemic zone for HBV infection, with an HBsAg carrier frequency of 2%-4% in the community.[4] HBV infection in India is responsible for 35%-60% of chronic liver disease and 60%-80% of HCC.[5] The prevalence of HCV is about 0.8– 1.5% in the general population and it has been implicated as the causative agent in 14-26% of chronic liver diseases in India.[6,7] Thus, a large part of the population is ‘at risk’ for developing chronic liver disease and, therefore, HCC. Additionally, due to the lack of screening programmes in the country, the majority of the patients here are still being diagnosed at an advanced stage of HCC.[8,9]
The existent curative treatment options like surgical resection, liver transplant and percutaneous ablation are applicable to patients with ‘early small HCC.’ Advanced disease at the outset precludes the use of radical therapy and therefore palliative therapy forms the mainstay of treatment in this group of patients.
Palliative therapy does not aim to cure but instead looks to improve survival and the quality of life. Previously, the only palliative treatment available for advanced HCC was chemotherapy or at best supportive therapy alone. The current era provides a plethora of options like transarterial chemoembolisation (TACE), oral chemotherapy and targeted molecular therapy. This review essays the historical perspective and the current status of palliative treatment in advanced HCC.
Transarterial chemotherapy/ embolisation
The liver has a dual blood supply, from the portal vein and the hepatic artery. The portal vein is responsible for supplying the majority of blood to the liver (75%-80%), with the hepatic artery providing 20%-25%. This balance is significantly altered in HCC where the hepatic artery becomes the sole supplier of blood to the tumour. The rationale of TACE is based on this and thus the hepatic artery is used as a medium to treat HCC. Various forms of intra-arterial treatment options are available, like transarterial embolisation (TAE), transarterial chemotherapy (TAC) and transarterial chemoembolisation (TACE).
Transarterial embolisation (TAE) is done using sterile absorbable gelatin sponge (Gelfoam), stainless steel coils, polyvinyl alcohol (PVA) particles (Ivalon), degradable starch microspheres (DSM), or embospheres depending on the size of the artery being embolised. Stainless steel coils and Ivalon particles cause permanent occlusion of the hepatic artery, whilst Gelfoam produces temporary occlusion.
In transarterial chemotherapy (TAC), only the chemotherapeutic agents are injected though the hepatic artery with no subsequent embolisation of the hepatic artery. Recently TACE has replaced TAE and TAC.
TACE is the most commonly used primary treatment for advanced HCC, as an adjunct to surgical resection, bridgingtherapy before transplantation and in conjunction with other ablative therapies as part of multimodality therapy.[10,11] The procedure of TACE includes intra-arterial administration of chemotherapeutic agents followed by the administration of the temporary embolising agent. The procedure can be performed in cases of impaired liver function (Child-Pugh’s A /B) and is also effective for multiple and large lesions. TACE has shown significant tumour response in 17%-61.9% cases, but poor complete tumour response (0-4.8%) due to residual viable tumour following the procedure.[12] There is limited effect of TACE in cases where capsular invasion, extra capsular growth or vascular invasion is present. Indications of TACE should be limited to situations of advanced HCC where surgery or ablation cannot be performed.[13]
Doxorubicin, mitomycin and cisplatin are the common anti-tumour drugs used alone or in combination. No standardised protocol exists with regard to the choice of chemotherapeutic agent, dosage, dilution, rate of injection and the optimal re-treatment strategy. In the same way, there is no standard embolising agent and quantity. Further RCTs are needed to assess the optimal chemotherapeutic agent and the treatment schedule.
The results of TACE have been extensively studied. A review from Japan reported that TACE was not indicated as first line treatment for early stages of HCC since it had worse outcome than surgery or percutaneous ablation.[14] The best candidates for TACE are patients with preserved hepatic function (Child-Pugh’s A), asymptomatic multinodular tumours, tumour without vascular invasion or extahepatic spread [Barcelona Clinic Liver Cancer Classification (BCLC) B].[15,16] A study by Georgiades et al17 reported that portal vein thrombosis should not be considered a contraindication to TACE, whereas patients with hepatic failure (Child-Pugh’s B-C) should be excluded since the ischemic insult may result in severe complications.
There are a number of factors associated with favourable prognosis, such as tumour diameter less than 5 cm, less than 50% replacement of liver by tumour tissue and unilobar tumour. Other prognostic factors include the AFP level, type of HCC, number of tumour nodules, portal vein thrombosis, presence of tumour capsule, and degree of lipiodol retention.[15,16,17,18,19,20,21,22,23] Large HCC, with poor baseline liver function, has shown least benefit from TACE.
TACE is a safe procedure with few complications, the most common being the post-embolisation syndrome (80- 90%) showing variable presentation like fever, abdominal pain, nausea, vomiting, leucocytosis, and increased liver enzymes. This syndrome is self-limiting and treated symptomatically in most patients, and the severity decreases with subsequent TACE. The benefits of the procedure should not be offset by treatment-induced liver or renal derangement.
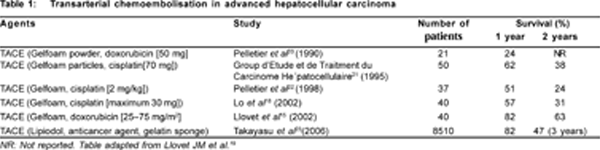
The impact of TACE for palliation of unresectable HCC has been depicted in several RCTs (Table 1). In 3 RCTs (systematic review, meta-analysis), TACE demonstrated greater survival benefit over supportive care.[15,16,18,19,20,21,22] Llovet et al[15] reported survival advantage at 1 and 2 years as 82% and 63% for chemoembolisation, and 63% and 27% for supportive care (p=0.009). Marked tumour response, with significant survival benefit in the chemoembolisation group (1 year, 57%; 2 years, 31%; 3 years, 26%) compared to the control group (1 year, 32%; 2 years, 11%; 3 years, 3%; p=0.002) has been documented.[18]
The largest cohort study of TACE for unresectable HCC on 8510 patients depicted a median survival of 34 months and survival benefit at 1, 3, 5 and 7 year as 82%, 47%, 26% and 16%, respectively with negligible procedure-related mortality (0.5%).[23] The degree of liver damage, TNM stage and á-fetoprotein values were independent predictors of patient survival.
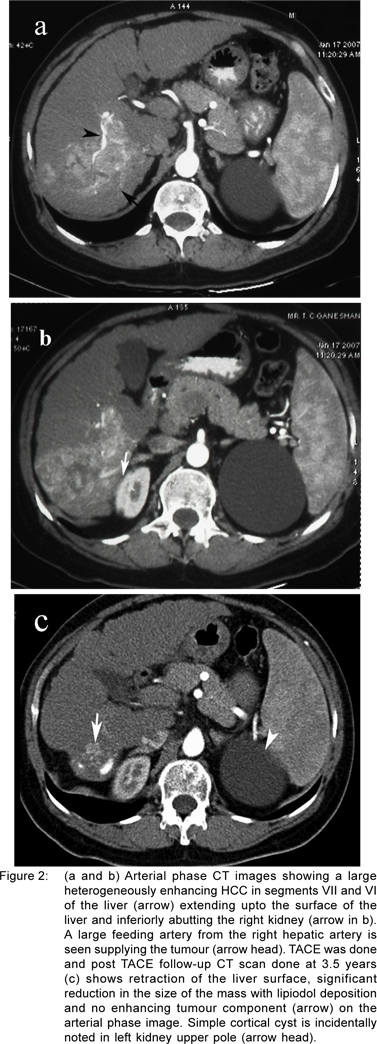
At our hospital, the All India Institute of Medical Sciences (AIIMS), TACE is being performed routinely in BCLC (Barcelona Clinic Liver Cancer) B and C cases and in rare cases of BCLC A (unsuitable for surgery or ablation). Doxorubicin 50 mg and cisplatin 100 mg are mixed with 10 ml of contrast media and 10-20 ml of lipiodol and the mixture is injected through the hepatic artery. This is followed by gelfoam embolisation. All patients are followed up by multi-phase CT study at 1, 3 and 6 months following the procedure. So far we have analysed the data of a total of 60 patients (56 male and 4 female) who underwent 111 sessions of TACE (Child’s A 42, Child’s B 18). The mean+SD follow up period following TACE was 3.5 months+13.6 months, median 8.8 months (interquartile range 4.3-19.4). The complications we encountered following TACE were deranged renal function tests in 15% and fever in 11%. The local response included freedom from disease in 28%, recurrence in 23%, fresh lesions in 28% and in 20% the response was not assessed due to the patients’ inability to undergo CT scan (Figures 1a-c and 2a-c). Patients with smaller HCC experienced better survival than patients with larger HCC (p=0.01). The two-year survival of patients with Child’s A tumours was higher than Child’s B patients though not statistically significant (51% vs. 30%, p=0.11). The cumulative survival at 1 and 2 years is 61% and 46%, respectively which compares well with studies from developed countries.[24]
In an effort to improve the anti-tumoural activity and clinical benefits following chemoembolisation, a new strategy of employing the drug eluting beads (DEB) has been tried successfully. Various concentrations of chemotherapeutic drugs like doxorubicin, epirubicin etc are available in the form of beads which when administered cause slow and sustained release of the drug, producing longer contact with the tumour and resulting in lesser systemic complications. A response rate of 75% and 1- and 2-year survival rates of 92.5% and 88.9%, respectively with DEB containing doxorubicin (150 mg) have been documented. Despite the high concentration of drug, systemic complications were negligible due to its slow release over a 1-week period.[25] However, we need more experience from other centres as well.
Transarterial radionuclide therapy (TART)
TART, also known as selective internal irradiation, is the delivery of radio-isotopes to the tumour through the hepatic artery similar to TACE. These radio-isotopes deliver the calculated radiation dose to the tumour with as minimal a dose as possible to the normal non-tumour liver parenchyma. The selection criteria for TART includes patients of unresectable HCC without extra-hepatic disease, and satisfactory liver function.[26]
Multiple radioactive substances have been tested which include Iodine-131 (131I)-lipiodol, rhenium-188, glass-based and resin-based 90Y microspheres. 131I-lipiodol therapy is a safe and effective palliative treatment for unresectable HCC. The overall survival benefits of 131I-lipiodol are 69%, 38%, 22%, 14%, at 6-months, 1, 2, 3 and 4 years, respectively.[26] RCTs have shown better survival rates in patients treated with Iodine- 131 (131I)-lipiodol than those treated with medical support group (3-, 6- and 9-month survival 71%, 48%, and 7%, respectively with 131I-lipiodol vs. 10%, 0% and 0%, respectively in medical support group).[27] Additionally, better patient tolerance and fewer vascular complications are observed without much survival benefit on comparison with TACE.[28] However, yet again our experience in this area is confined.
Two types of Yttrium-90 (90Y) microspheres, glass-based and resin-based 90Y microspheres are used in TART and at present this is still experimental. It causes lesser adverse effects due to the slow release of the radiopharmaceutical. No life-threatening complications have been encountered. The phase 2 study for HCC with and without portal vein thrombosis has shown the majority of adverse events in patients with main portal vein thrombosis and cirrhosis.[29] Studies have also shown reduction in tumour size, and tumour down-staging, thereby making feasible curative options like resection, RFA or transplantation. Improvement in the overall median survival rates have also been documented.[29,30]
Rhenium188 too has been successfully used in inoperable HCC. It is well tolerated and appears to be safe and effective in a selected group of patients. A multicentre clinical trial including our centre showed survival rates at 6, 9, 12, 24, and 36 months in patients with objective tumour response as 100%, 95%, 90%, 58%, and 30%, respectively, with a median survival of 980 days.[31] Another multicentre trial showed overall survival rate of 46% and 23% at 1 and 2 years.[32] There is paucity of data available and hence further research needs to be undertaken in this area as well.
Systemic and targeted therapy
HCC is highly refractory to cytotoxic systemic therapy partially due to its tumour biology, pharmacokinetic properties, and both intrinsic and acquired drug resistance. The development of resistance may be due to p53 mutation by hepatitis viruses and chemicals during molecular pathogenesis of HCC. Since chemotherapeutic agents require p53 to induce apoptosis, tumours with a disruption in the p53 pathway are thus resistant to chemotherapy.[33,34] In addition, mutations have also been found in p73, APC, Rb, c-myc, and cyclin DJ.[35] Over-expression of DNA topoisomerase in HCC may account for its resistanceto chemotherapy.[36]
In patients with cirrhosis, the liver function is significantly reduced which affects the absorption, plasma protein binding, distribution and renal excretion of drugs. Therefore, cirrhosis has a significant impact on the pharmacokinetics of systemic therapy for HCC. Intrinsic drug resistance of HCC cells is mediated by multiple proteins includes MDR1, p-glycoprotein, glutathione-S-transferace, heat shock proteins, multi-drug resistance protein, and co-expression of p53 and pglycoprotein. [37,38,39] Therefore it is reasonable to attempt avoiding these pathways in designing novel therapies. Several randomised, controlled studies have tested systemic treatments for HCC.
Systemic chemotherapy
A large number of studies have been performed with a single chemotherapeutic agent. Table 2 shows some of the studies that use a single agent in advanced HCC. Perhaps doxorubicin is the most commonly used agent in HCC, used alone or in combination with other agents. Reports of the initial study using single agent doxorubicin revealed encouraging results,[40] but the subsequent larger study by Sciarrino et al[41] failed to show any response. Recent large phase III studies with doxorubicin in the control group have shown survival rates of 8 months and a response rate of 4%.[42] Numerable complications are encountered with doxorubicin, commonly, hematologic and gastrointestinal. Febrile neutropenia (17%), neutropenia (63%), thrombocytopenia (24%) and diarrhoea (7%) preclude the use of doxorubicin.[40,41,42]
Other single agent chemotherapeutic agents, such as pegylated liposomal doxorubicin, cisplatin, capacitabine, 5- fluorouracil, etoposide have been tested with disappointing response rates and no survival benefits. In the same way, low response rates are shown by the newer generation chemotherapeutic agents like gemcitabine, paclitaxel, mitoxantrone and irofulven.[43,44,45,46,47,48,49] (Table 2)
Many researchers have tried combination systemic chemotherapy also in advanced HCC and the results from the recent studies are summarised in Table 3. Although some of them have shown promising response in phase II studies,[54] some of the studies failed to show survival benefits in phase III studies[57] and/or most of these have not been validated in large randomised phase III trials.[55,56]
Combination of epirubicin and etoposide has shown minimal anti-tumour activity with acceptable toxicity. The median overall survival is 6.4-10 months and response rate of 32 to 39%.[55,56]
Combination chemotherapy using interferon, cisplatin, doxorubicin, and 5-fluorouracil (PIAF) have shown the most promising results in both phase II and III trials.[52,53] Similar results have also been seen using Gemcitabine and Oxaliplatin (GEMOX) and in combination of GEMOX with cetuximab. The former demonstrated a clinical response rate of 18% with acceptable toxicity and median progression-free and overall survival time of 11.5 months.[58,59]
Other single agents like interferon, and the somatostatin analogues, octreotide and lanreotide, have failed to show survival benefit in the treatment of advanced HCC; they did however show some benefit in combination therapy.[51]
Thalidomide has been tested extensively in HCC. Itsmechanism of action is poorly understood and complex, and probably involves its anti-angiogenic action by modulating factors like tumour necrosis factor-á, interferon, interleukins 10 and 12, cyclooxygenase-2. Recent phase II studies of thalidomide either as single agent or in combination with interferon or epirubicin have shown limited activity in HCC with response rate of 3.1 to 6.3 and median survival of 2.7 to 6.8 months.[62]
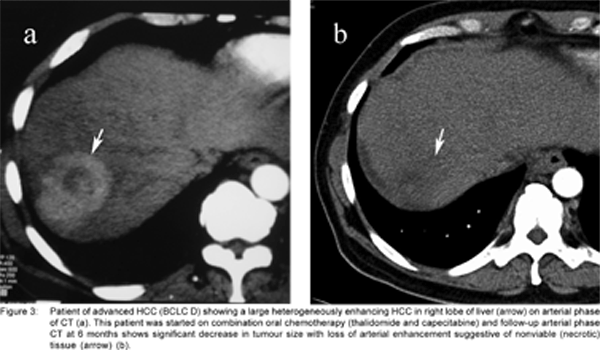
At AIIMS, we are conducting an RCT on stage D patients of HCC, comparing combination therapy of oral thalidomide and capecitabine with supportive therapy. So far 54 patients have been recruited into the trial. Clinical follow up is done at monthly intervals. Radiological follow up is done at 1, 3 and 6 months by multiphase CT in both groups. The final outcome is local response on multiphase CT and survival rate. In patients who showed clinical response following treatment with combination chemotherapy, we also noticed the appearance of necrosis and reduction in the size of the tumour on multi phase CT (Figures 3 a &b).
Presently, there is no single or combination drug regimen that can be clearly defined as the standard for treating advanced HCC. Heterogeneity of disease, patient selection bias and greater toxicity are the limiting factors in the use of combination chemotherapy.
Hormonal therapy
Hormonal agents were used to treat advanced HCC due to its expression of estrogen, progesterone and androgen receptors. In the past, tamoxifen was commonly used from amongst the various hormonal agents for the treatment of advanced HCC, due to its good tolerability and oral administration. A recent systematic review and meta-analysis conducted by Di Maio M et al [63] has shown disappointing results concerning the efficacy of hormonal treatment in advanced HCC. This raises serious doubts regarding the relevance of sex hormones mediated pathways in the clinical course of HCC. With the available clinical evidence, the author does not suggest hormone therapy as part of the current standard management of advanced HCC.
Targeted therapy
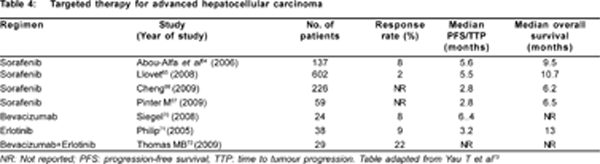
Understanding the role of molecular pathogenesis of HCC has lead to better management with the introduction of molecular targeted therapy. There are many key carcinogenic pathways which play a major role in the development of HCC, the pathway responsible for hepato-carcinogenesis is difficult to assess. By targeting a few of the pathways like the anti- angiogenic pathway, Raf/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), epidermal growth factor receptor-1 (EGFR) and phosphatidylinositol-3- kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathways, exciting clinical benefits have been documented.[51] Most of these are focused mainly on inhibiting tumour angiogenesis and/or inhibiting the tumour growth pathway. Table 4 shows the recent trials of targeted chemotherapy in advanced HCC.
Sorafenib, an oral multikinase inhibitor, is the only drug currently approved in the management of advanced HCC. It targets multiple pathways, tyrosine kinase, VEGR-2, VEGR-3 (vascular endothelial growth receptor), and PDGF receptor á (platelet derived growth factor) which result in its antiangiogenic property and it blocks tumour proliferation by targetting the Raf/MAPK/ERK signaling pathways and cytokines like Flt3, c-KIT and p38a. It has undergone extensive investigation in phase I, II, III trials.[64,65,66.67] The majority of patients evaluated with sorafenib belong to Child-Pugh A cirrhosis with favourable general condition. The SHARP study conducted in western countries where chronic HCV and alcohol are the major risk factors for HCC, and the oriental study in the hepatitis B endemic Asian population revealed sorafenib as effective and well tolerated for the treatment of advanced HCC in both regions.[65,66]
Recently the efficacy of sorafenib in patients with multifocal hepatocellular carcinoma (HCC) in advanced liver cirrhosis has been evaluated. The overall survival time was better in patients with well-preserved liver function and lower BCLC stage than Child-Pugh B cirrhosis. The life expectancy was limited due to severe underlying disease and drug-related complications in patients with Child-Pugh C cirrhosis.[67]
The US Food and Drug Administration (FDA) has independently analysed the overall survival of patients treated with the best supportive care plus sorafenib compared with placebo in advanced HCC. The median survival advantage of sorafenib was 10.7 months vs. 7.9 months for placebo. Sorafenib has received FDA approval in treatment of advanced HCC mainly due to its survival advantage and its tolerability.[68] Drug-related adverse events were diarrhoea (39%) reported in the SHARP study,[65] hand-foot skin reaction (10·7%), diarrhoea (6·0%), and fatigue in an oriental study, [66]
Researchers have designed a large multicentre phase III RCT on TACE combined with sorafenib vs. TACE plus placebo in patients with HCC to reveal the potential superiority of combined TACE plus sorafenib over TACE alone.[68,69]
Other targeting agents like sunitinib, a multi-target tyrosine kinase inhibitor, cetuximab, targeting the epidermal growth factor pathway, and bevacizumab are being studied in phase II and III trials. The results are eagerly awaited to guide the future use of these drugs in the treatment of advanced HCC.[70,71,72]
Multimodality approaches
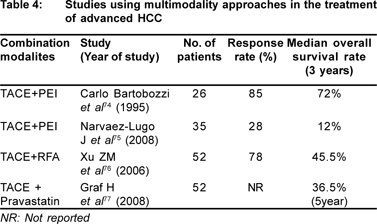
A combination of various treatment modalities are being resorted for treating large tumours in order to achieve higher efficiency. Studies have used treatment options of locoregional and systemic treatment modalities like TACE, percutaneous ethanol injection (PEI), percutaneous acetic acid injection, radiofrequency ablation (RFA), oral chemotherapy and targeted therapy in various combinations for treating larger tumours (Table 4). Combination modalities have shown better survival rates.[73,74,75,76,77]
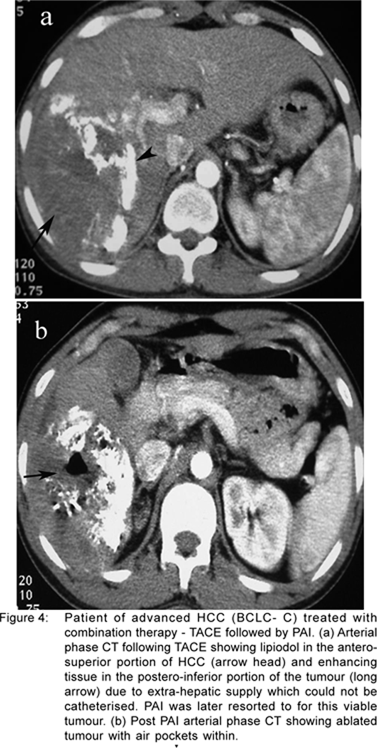
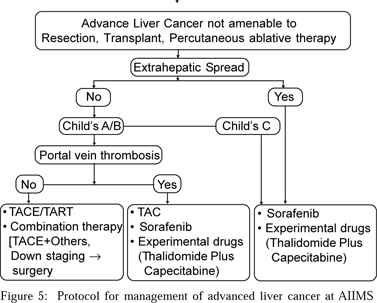
At our centre, we combine TACE with percutaneous acetic acid injection (PAI) or RFA in large unresectable tumours (Figuress 4 a & b). Patients of BCLC B/C who are subjected to TACE as the first line treatment, are subsequently switched to combination therapy (PAI or RFA) if they have the following a) presence of additional extrahepatic arterial supply to the HCC which is difficult to catheterise, b) poor response following TACE due to the inherent tumour biology, c) inability to repeat TACE due to blocked hepatic artery or deteriorating hepatic or renal function. There is no recommended protocol for this. The decision is taken by the treating team depending on the availability of the treating modalities and the patient’s condition. A combination of TACE with a wide variety of systemic chemotherapeutic drugs has been tested and shows conflicting results. An RCT comparing TACE with TACE and oral chemotherapy has also been undertaken at our centre. A flow chart (Figure 5) outlines the protocol of management of advanced liver cancer undertaken at the All India Institute of Medical Sciences.
Tumour downstaging for advanced HCC
Tumour downstaging followed by salvage surgery is a new concept in the intention to treat patients of advanced andunresectable HCC. The rationale behind tumour downstaging is the disappearance of some of the tumour nodules and venous thrombi, shrinking of large HCCs, and enlargement of the non-tumorous part of the liver after administration of the downstaging regimen. The downstaging regimens include TACE, TART, PEI and systemic chemo-immunotherapy. However, a more effective regimen is still unknown. The patient selection includes the general condition of the patient, tumour staging, liver function, the patient’s preference, and the availability of expertise. The contraindications to this treatment are no different from that of partial hepatectomy. There should be an aggressive surgical approach followed by repeated radiological assessment of tumour shrinkage. The outcome of salvage surgery following tumour downstaging has a longterm survival rate (5-year survival 24.5% to 57%). Minimal response to the downstaging regimen and second tumour development in cirrhotic liver are the main problems with tumour downstaging.[78]
Cytoreductive surgery
Tumour debulking is removal of macroscopic tumours leaving behind microscopic foci and preserving the functional liver as much as possible. There are few combination adjuvant treatment modalities used along with partial hepatectomy in unresectable and advanced HCC. These include intra operative local ablation of small tumours in liver remnant, postoperative adjuvant regional chemotherapy, and both of these. There is increased sensitivity of the remaining tumour to chemotherapy, and marked improvement in patient symptoms and quality of life following cytoreductive surgery. The non-randomised studies have shown 3-year survival rates of 7%-48.7%.[78] The disadvantages with cytoreductive surgery are tumour growth despite adjuvant therapy, and delay in other beneficial non-surgical options.
Conclusion
Treatment of advanced HCC remains a challenge. Advanced HCC is not uncommon at diagnosis in developing countries where routine screening is not performed. It is therefore imperative to develop effective and affordable therapeutic treatment strategy for advanced disease. Careful individualised treatment with the available therapeutic modalities can enable favourable outcome in this group of patients.
TACE has been extensively studied with multiple chemotherapeutic agents with conflicting results. Most of the RCTs have shown survival benefit but a standard protocol is yet to be developed with further RCTs. TART using Iodine-131, Yttrium-90 and rhenium-188 has steadily shown results comparable to TACE. Hepatic resection in advanced HCC has shown disappointing results due to its limitations with respect to vascular invasion, tumour rupture and dissemination during surgery. However, later studies have shown survival benefits with adjuvant therapy.
So far no systemic chemotherapeutic agent has shown higher survival rates. Single agent sorafenib has shown promising results with 3-month survival benefits and good tolerability. Although it is the only chemotherapeutic drug approved by FDA in the treatment of advanced HCC, there is no data currently available regarding the cost effectiveness of sorafenib, which precludes its use in developing countries. There are multiple ongoing phase II and III studies testing various single and combination forms of therapy, and also the newer targeted agents are being tried in combination with TACE hoping to produce survival benefits in advanced HCC.
The multimodality approach is the need of the hour and has shown better survival benefits than single modality treatment. Studies combining sorafenib with other established palliative modalities are on the way. Researchers need to unravel the underlying hepatocarcinogenesis and key molecular targets for development of more effective chemotherapeutic agents in order to improve survival in advanced HCC patients.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108.
2. Pisani P, Parkin DM, Ferlay J. Estimates of the world wide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993;55:891–3.
3. Tanaka H, Hiyama T, Tsukuma H, Okubo Y, Yamano H, Kitada A, et al. Prevalence of second generation antibody to hepatits C antibody among voluntary blood donors in Osaka, Japan. Cancer Causes Control. 1994;5:409–13.
4. Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India 2006; 19(4):203–17.
5. Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology. 2002;62 Suppl 1:8–17.
6. Ramesh R, Munshi A, Panda SK. Prevalence of hepatitis C virus antibodies in chronic liver disease and hepatocellular carcinoma patients in India. J Gastroenterol Hepatol. 1992;7:393–5.
7. Panigrahi AK, Panda SK, Dixit RK, Rao KV, Acharya SK, Dasarathy S, et al. Magnitude of hepatitis C virus infection in India: prevalence in healthy donors, acute and chronic liver disease. J Med Virol. 1997;51:167–74.
8. Sarin SK, Thakur V, Guptan RC, Saigal S, Malhotra V, Thyagarajan SP, et al. Profile of hepatocellular carcinoma in India. An insight into the possible etiologic associations. J Gastronterol Hepatol. 2001;16: 666–73.
9. Batra Y, Gulati M, Paul SB, Acharya SK. Clinical profile and results of therapy in patients of HCC at a tertiary care center in India. J Gastroenterol Hepatol 2004;19: A799.
10. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17.
11. Seror O, N’Kontchou G, Haddar D, Dordea M, Ajavon Y, Ganne N, et al. Large Infiltrative Hepatocellular Carcinomas: Treatment with Percutaneous Intraarterial Ethanol Injection Alone or in Combination with Conventional Percutaneous Ethanol Injection. Radiology 2005;234:299–309.
12. Jansen MC, van Hillegersberg R, Chamuleau RA, van Delden OM, Gouma DJ, van Gulik TM. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol. 2005;31:331–47.
13. Masuzaki R, Omata M. Treatment of Hepatocellular carcinoma. Indian J Gastroenterol. 2008;27:113–22
14. Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802.
15. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte Jet al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9.
16. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188.
17. Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–9.
18. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71.
19. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–42.
20. Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, et al. A randomized trial of hepatic arterial hemoembolization in patients with unresectable hepatocellular carcinoma. Hepatol. 1990;11:181–4.
21. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256–61.
22. Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–34.
23. Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
24. Paul SB, Gamanagatti SR, Sreenivas V, Madan K, Baba CS, Gulati MS, Gupta AK and Acharya SK. Efficacy of transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC): experience from a tertiary care centre in India. J Gastroenterol Hepatol. 2008;23:A64
25. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81.
26. Lau WY. Radiotherapy with emphasis on radioembolization for liver tumors. In: Blumgart LH & Fong Y (2nd eds). Surgery of the Liver and Biliary Tract. W. B. Saunders London; 2000:1545–1564.
27. Raoul JL, Guyader D, Bretagne JF, Duvauferrier R, Bourguet P, Bekhechi D, et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131- iodized oil versus medical support. J Nucl Med. 1994;35:1782–7.
28. Raoul JL, Guyader D, Bretagne JF, Heautot JF, Duvauferrier R, Bourguet P, et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26:1156– 61.
29. Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, et al. Yttrium-90 microspheres TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572–86.
30. Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–39.
31. Kumar A, Srivastava DN, Chau TT, Long HD, Bal C, Chandra P, et al. Inoperable hepatocellular carcinoma: transarterial 188Re HDDlabeled iodized oil for treatment—prospective multicenter clinical trial. Radiology. 2007;243:509–19.
32. Bernal P, Raoul JL, Stare J, Sereegotov E, Sundram FX, Kumar A,, et al. International Atomic Energy Agency-Sponsored Multination Study of Intra-Arterial Rhenium-188-Labeled Lipiodol in the Treatment of Inoperable Hepatocellular Carcinoma: Results With Special Emphasis on Prognostic Value of Dosimetric Study. Semin Nucl Med. 2008;38:S40–5.
33. Fabregat I, Roncero C, Fernández M. Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int. 2007;27:155–62.
34. Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–76.
35. Feitelson MA, Sun B, Satiroglu Tufan NL, Liu J, Pan J, Lian Z.. Genetic mechanisms of hepatocarcinogenesis. Oncogene. 2002;21:2593–604.
36. Watanuki A, Ohwada S, Fukusato T, Makita F, Yamada T, Kikuchi A, et al. Prognostic significance of DNA topoisomerase IIalpha expression in human hepatocellular carcinoma. Anticancer Res. 2002;22:1113–9.
37. Ng IO, Liu CL, Fan ST, Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am J Clin Pathol. 2000;113:355–63.
38. Park JG, Lee SK, Hong IG, Kim HS, Lim KH, Choe KJ, et al. MDR1 gene expression: its effect on drug resistance to doxorubicin in human hepatocellular carcinoma cell lines. J Natl Cancer Inst. 1994;86:700–5.
39. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
40. Johnson PJ, Williams R, Thomas H, Sherlock S, Murray-Lyon IM. Induction of remission in hepatocellular carcinoma with doxorubicin. Lancet. 1978;1:1006–9.
41. Sciarrino E, Simonetti RG, Le Moli S, Pagliaro L. Adriamycin treatment for hepatocellular carcinoma. Experience with 109 patients. Cancer. 1985;56:2751–5.
42. Gish RG, Porta C, Lazar L, Ruff P, Feld R, Croitoru A, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol. 2007;25:3069–75.
43. Hong RL, Tseng YL. A phase II and pharmacokinetic study of pegylated liposomal doxorubicin in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2003;51:433–8.
44. Fuchs CS, Clark JW, Ryan DP, Kulke MH, Kim H, Earle CC, et al. A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2002;94:3186–91.
45. Falkson G, Ryan LM, Johnson LA, Simson IW, Coetzer BJ, Carbone PP, et al. A random phase II study of mitoxantrone and cisplatin in patients with hepatocellular carcinoma. An ECOG study. Cancer. 1987;60:2141–45.
46. Patt YZ, Hassan MM, Aguayo A, Nooka AK, Lozano RD, Curley SA, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101:578–86.
47. O’Reilly EM, Stuart KE, Sanz-Altamira PM, Schwartz GK, Steger CM, Raeburn L, et al. A phase II study of irinotecan in patients with advanced hepatocellular carcinoma. Cancer. 2001;91:101–5.
48. Chao Y, Chan WK, Birkhofer MJ, Hu OY, Wang SS, Huang YS, et al. Phase II and pharmacokinetic study of paclitaxel therapy for unresectable hepatocellular carcinoma patients. Br J Cancer. 1998;78:34–9.
49. Falcon-Lizaraso S Leon Chong J, Perazzo F et al. Phase II trial of every 2 weeks dosing of irofulven (IROF) in patients (pts) with unresectable hepatocellular carcinoma (HCC): preliminary results. Proc Am Soc Clin Oncol 2004;22:4083a.
50. Leung TW, Feun L, Posey J et al. A phase II study of T138067- sodium in patients (pts) with unresectable hepatocellular carcinoma (HCC). Proc Am Soc Clin Oncol 2002;20:572a.
51. Andrew X. Zhu. Systemic Therapy of Advanced Hepatocellular Carcinoma: How Hopeful Should We Be? Oncologist. 2006;11:790– 800.
52. Lee J, Park JO, Kim WS, Park SH, Park KW, Choi MS, et al. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:385–90.
53. Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, et al. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421–7.
54. Bobbio-Pallavicini E, Porta C, Moroni M, Bertulezzi G, Civelli L, Pugliese P, et al. Epirubicin and etoposide combination chemotherapy to treat hepatocellular carcinoma patients: a phase II study. Eur J Cancer. 1997;33:1784–8.
55. Lee JL, Ryu MH, Chang HM, Kim TW, Lee SS, Sym SJ, et al. Efficacy and safety of epirubicin and etoposide combination chemotherapy in advanced hepatocellular carcinoma: a retrospective analysis. J Gastroenterol Hepatol. 2008;23:811–6.
56. Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676–81.
57. Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, et al. A randomized phase III study of doxorubicin versus cisplatin/ interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–8
58. Louafi S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109:1384–90.
59. Asnacios A, Fartoux L, Romano O, Tesmoingt C, Louafi S S, Mansoubakht T, et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer. 2008;112:2733–9.
60. Ikeda M, Okusaka T, Ueno H, Takezako Y, Morizane C. A phase II trial of continuous infusion of 5-fluorouracil, mitoxantrone, and cisplatin for metastatic hepatocellular carcinoma. Cancer. 2005;103:756–62.
61. Yuan JN, Chao Y, Lee WP, Li CP, Lee RC, Chang FY, et al. Chemotherapy with etoposide, doxorubicin, cisplatin, 5-fluorouracil, and leucovorin for patients with advanced hepatocellular carcinoma. Med Oncol. 2008;25:201–6.
62. Yau T, Chan P, Epstein R, Poon RT. Evolution of systemic therapy of advanced hepatocellular Carcinoma. World J Gastroenterol. 2008;14:6437–41.
63. Di Maio M, Daniele B, Pignata S, Gallo C, De Maio E, Morabito A, et al. Is human hepatocellular carcinoma a hormone-responsive tumor?. World J Gastroenterol. 2008;14:1682–9.
64. Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300.
65. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
66. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
67. Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, et al. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70–6.
68. Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95–100.
69. Hoffmann K, Glimm H, Radeleff B, Richter G, Heining C, Schenkel I,, et al. Prospective, randomized, double-blind, multi-center, Phase III clinical study on transarterial chemoembolization (TACE) combined with Sorafenib® versus TACE plus placebo in patients with hepatocellular cancer before liver transplantation – HeiLivCa. BMC Cancer. 2008;8:349
70. Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–8.
71. Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, e al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657–63.
72. Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–50.
73. Yau T, Chan P, Epstein R, Poon RT. Management of advanced hepatocellular carcinoma in the era of targeted therapy. Liver Int. 2009;29:10–7.
74. Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S,et al. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812–8.
75. Narvaez-Lugo J, Cáceres WW, Toro DH, Pérez-González MR, Almodovar AA, Maldonado-Mercado AM, et al. Transcatheter arterial chemoembolization and percutaneous ethanol injection for Hepatocellular carcinoma: a retrospective review of the Veterans Affairs Caribbean Healthcare System. Cancer Control. 2008;15:80–5.
76. Xu ZM, Wang JH, Zhen ZJ, Chen HW, Cui WZ. Percutaneous radiofrequency ablation combined with transcatheter arterial chemoembolization and percutaneous ethanol injection for recurrent small hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1626–8.
77. Graf H, Jüngst C, Straub G, Dogan S, Hoffmann RT, Jakobs T, et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion. 2008;78:34–8.
78. Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2008;7:237–57.
|
|
|
 |
|
|