|
|
|
|
 |
 |
| |
 |
|
|
Original Articles |
|
|
|
|
|
Keywords :
|
|
|
Ramesh Roop Rai
Department of Gastroenterology,
SMS Medical College,
Jaipur, India.
Corresponding Author:
Dr. Ramesh Roop Rai
Email: rameshroop@gmail.com
DOI:
http://dx.doi.org/
Abstract
Aim: In an outbreak of hepatitis E affecting 859 individuals, we evaluated the titres of serological markers (IgM anti-HEV and IgG anti-HEV) and hepatitis E virus (HEV) RNA by reverse transcriptase polymerase chain reaction.
Methods: Serological markers for acute hepatitis were evaluated in 294 icteric patients (Group A) and 300 apparently healthy controls (Group B). HEV RNA was measured by RT nPCR in 19 patients in the first week of illness in patients with negative IgM anti-HEV.
Findings: None of the patients were positive for hepatitis A or B. In Group A, IgM anti-HEV was positive in 80.2%, 71.4% and 26.8% and IgG anti-HEV was positive in 58.3%, 77.1% and 86% of patients who were in their first, second and third weeks of illness, respectively. In Group A, amongst the 19 IgM anti-HEV negative patients in their first week of illness, 16 were positive for HEV RNA. In Group B 63.6% cases were positive for IgM anti-HEV. In the same village there had been a similar epidemic 4 years ago; none of the 93 patients traced from that time developed acute hepatitis during the present epidemic and all demonstrated the presence of IgG anti-HEV. This suggests that IgG anti-HEV was perhaps protective.
Conclusion: During the first week of illness patients may display HEV viremia while testing negative for IgM and IgG anti-HEV. The presence of IgG anti-HEV may play a protective role against HEV infection and in the absence of IgM may help in diagnosing acute hepatitis E. Over 3 weeks of illness the IgM anti-HEV titres fall progressively whilst IgG anti-HEV titres gradually rise.
|
48uep6bbphidvals|188 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Hepatitis E virus (HEV) is responsible for epidemic and endemic hepatitis in the developing world.[1,2] HEV is transmitted by the faeco-oral route, and drinking water supply contaminated with faeces has been documented as the main cause of epidemic hepatitis. However, person-to-person transmission of HEV is infrequent. During epidemics, anicteric hepatitis is generally more frequent than icteric hepatitis.[1,2,3,4,5,6,7,8,9,10] Usually HEV causes mild, self-limiting hepatitis without any chronic sequelae. However, HEV has been associated with severe liver disease particularly in pregnant women. Also pregnant women in their second and third trimesters contract HEV infection more frequently (12-20%) than non-pregnant women and men (2-4%).[1,2,3,4,5,6,7,8,9,10,11]
During HEV epidemics, the primary disease attack rate lies between 1% and 15% and the secondary attack rate amongst household contacts of HEV infected individuals has been documented from 0.7% to 2%.[10,12,13,14,15]
Limited information is available on the dynamics of IgM and IgG anti-HEV in hepatitis E epidemics at separate points in the illness,[15,16,17] prevalence of anti-HEV antibodies in healthy contacts of index patients and the role of HEV RNA in the diagnosis of serologically negative patients. The aim of this study was to evaluate the serological and molecular markers of hepatitis E virus in an epidemic in Khanpur, Rajasthan.
Methods
Pilot survey: On the 26th of February 2006, the local health authorities informed us that the incidence of jaundice had increased in Khanpur town (Jhalawar district), Rajasthan. A team of specialists was sent to the site to investigate the clinical presentation, attack rate, source of infection, serological markers, and morbidity and mortality associated with the affliction in the affected population. Eight investigative teams were made, each consisting of one doctor pecialists from SMS Medical College, Jaipur and Kota Medical College), two nursing staff and two paramedical staff. These teams conducted a house-to-house survey using a set questionnaire, which included age, sex, time of onset of illness and source of water supply. Acute hepatitis was clinically defined as yellowish discolouration of the conjunctiva or passing of high-coloured urine, preceded by typical prodromal symptoms viz. malaise, fever, generalised body aches, nausea and vomiting. The team evaluated those persons who had jaundice or had suffered from jaundice a few days before and had recovered. Persons who had chronic liver disease or extra hepatic biliary obstruction were excluded from the study. The survey proforma collected the following data from each house: the number of family units, the number of family members in each unit, the source of drinking water supply for each family unit, and the number of icteric patients in each unit. Each jaundiced patient was further evaluated by a team of 3 gastroenterologists between the 6th and 10th of March 2006. The patients were labeled as Group A: icteric patients, Group B: family contacts of icteric patients or healthy neighbors, Group C: patients who suffered from jaundice in a previous epidemic in the same town four years ago in 2002. All the people reporting to the primary health centre (PHC) were clinically evaluated and serum samples drawn for various tests viz. serum bilirubin, aspartate aminotransferase, alanine aminotransferase, HBsAg, IgM anti-HBc, IgM anti-HEV and IgG, and IgM anti-HAV (commercial ELISA). A diagnosis of hepatitis E was based on the presence of IgM anti-HEV and the absence of IgM anti-HBc and IgM anti-HAV.
Village topography: Khanpur has a population of about 19,000 and is spread over approximately 1.5 square kilometres. The topography of the town is uneven, as it is a hilly area. All the houses are situated very close to each other and have narrow streets, joining the main street and there are blocked overflowing drains on either side of the road spilling wastewater from the houses and sewage of lavatories.
Sewage system: Most families let the faecal matter from their toilets pass directly in to the nearby open drains. Some families have septic tanks or only soakage pits within the premises of the house. Most of the sewage from these septic tanks drains in to the nearby open drains. This raw sewage gets collected in three or four pools. There is a large drain along the main street in to which all sewage and wastewater drains. Due to the uneven terrain of the area there is partial blockage of the drain at various places. Sewage water overflows and gets collected in low-lying areas.
Drinking water source: The entire village gets its water supply either from the government water supply or through hand pumps. The government water supply is from two overhead water reservoirs located at two ends of the town, overhead 1 and overhead 2. Overhead 1 receives water from two wells which are open, and are 60 metres deep with an additional boring of 60 metres. Overhead 1 receives water from well number 2 which is situated on the northern side very close (less than 10 metres) from a wastewater drain. On the 5th of February 2006, there was heavy rain. At that time due to over-flooding of this drainage system the sewage and wastewater found their way into the open well 2 thus infecting the whole water supply coming from overhead 1.
Hand pumps: There are 60 hand pumps in the town. Many hand pumps are very close to the drains and some appear to have been almost dug over the open drains.
Distribution system of water is through pipelines which were laid approximately 27 years ago and pass either below, within or along the major drains of the town. Water is supplied once a day for one hour and as the water pressure is often low, people have either broken the pipelines or collected water from a ditch, which is close to the sewage line, or installed inline booster pumps to gain more water. The pipelines, when examined, were rusted and broken at many places, and joints of pipes were loose. When the water rushed in, a negative pressure was created, sucking the sewage water into the pipelines, contaminating the water. Contamination from sewage into supply line well 2 occurred on the 5th and 6th of February 2006.
Water analysis: Water samples were collected from thetwo overhead water reservoirs and hand pumps. The samples were analysed for coliform count and chlorine content at the Public Health and Engineering Department (PHED) Department between the 20th of February and 26th of March 2006 at 3-day intervals.
Serological tests: A total of 859 jaundiced patients were included in the study. Amongst 859 patients, 294 patients underwent serological testing. This study also included 300 healthy family members who were asymptomatic and who had consumed the same drinking water. Blood samples were collected from both icteric and anicteric persons. All the serum samples included in this study were stored at -20°C till the serological investigations were carried out. Serum samples were examined for IgM anti-HEV and IgG anti-HEV, HBsAg, IgM anti-HBc and IgM anti-HAV antibodies using a commercially available ELISA kit (Biochem, Italy).
HEV RNA testing:19 blood samples were collected in patients who were negative for IgM anti-HEV within the first week of illness and were subjected to HEV RNA detection by nested reverse transcriptase polymerase chain reaction (nRTPCR). The RNA was extracted by the Trizole method. After cDNA synthesis using MMLV RT enzyme and random hexamer, PCR amplification was carried out using specific primers against the ORF1 gene, (sense: 5’-CGGGATCCACACACATCTGAGCTACATTCGTGAGCT-3’ antisense: 5’- CGGAATTCAAAGGCATCCATGGTGTTTGAGAATGAC-3’ for first cycle and sense: 5’- GGAATTCGACTCCACCCAGAATAACTT-3’ antisense: 5’-GGAATTCACAGCCGGCGATCAGGACAG-3’ for second cycle) which gave rise to a product of 343 bp visualized by agarose gel electrophoresis according to the protocol mentioned by Jameel et al (1992).[18]
Results
There were 859 patients amongst a total population of 19,000 that suffered from jaundice in the present epidemic; and the attack rate was 4.5% (859/19,000). Serological markers for acute hepatitis were evaluated in the 294 jaundiced patients from Group A and the 300 apparently healthy subjects from Group B. In both groups, markers for acute hepatitis A, acute hepatitis B and acute hepatitis C were negative. There were more male patients in both Groups A (n=204) and B (n=206). The maximum number of patients was in the age group of 15-30 years followed by the 31-45 years category (Table 1). The paediatric population (less than 15 years) was affected less than the adults; the number of children accounted for 13.1% of the total number of icteric patients. There were 39 pregnant women who suffered from hepatitis E and accounted for 4.5% of the total icteric population.
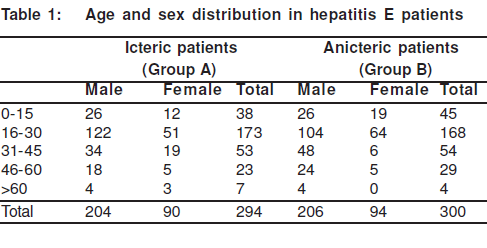
Serological data
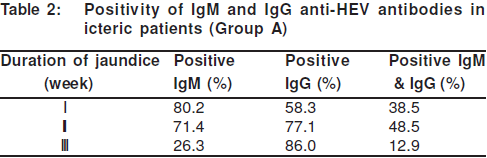
Seroprevalence of HEV was studied in 294 cases from Group A and 300 from Group B. (Table 2) In Group A, 96 patients were in the first week of illness, 105 were in the second week and 93 were in the third week of illness; positive IgM anti-HEV was seen in 77/96 (80.2%) patients who were in the first week of illness, 75/105 (71.4%) patients in the second week and 25/93 (26.8%) patients in the third week of illness. Anti-HEV IgG was detected in 56 (58.3%), 81 (77.1%) and 80 (86%) of the jaundiced patients who were in the first, second and third weeks of illness, respectively. Isolated IgG without IgM antibody was observed in 19 (19.8%), 30 (28.5%) and 68 (73.1%) icteric patients who were in the first, second and third weeks of illness, respectively. There were 19 patients in their first week of illness in whom IgM anti-HEV was negative, HEV RNA test was performed in this subgroup of patients, and HEV RNA was detected by nRT PCR in 16 (84%). However, both types of antibodies were detected in 37%, 51% and 12% of jaundiced patients in the first, second and third weeks of illness, respectively.
At the same time, subjects in Group B who were asymptomatic, residing in the same geographical area and consuming the same drinking water also had positive IgM anti-HEV in high numbers, i.e. 191/300 (63.6%). IgG anti-HEV was present in 25.6% cases and only 18% tested positive for both IgM and IgG antibodies. There was a steady fall in the ALT level during the course of illness in Group A patients. The ALT level was 2078.6±108.7, 1428.0±48.8 and 686.5±67.9 IU/L in the first, second and third weeks, respectively. Group B patients who were positive for IgM and IgG anti-HEV also showed a higher than normal level of ALT. ALT was 198.2±98.6, 116±44.8 and 43.0±36.9 IU/L in the first, second and third weeks,respectively. None of the patients in Group B developed jaundice.
Past epidemic
A similar epidemic of jaundice had occurred in the same area in 2002 in which there were 173 cases, 93 of these cases were traceable and they formed Group C. Of these 93 patients, 76 patients were positive for IgG anti-HEV; none demonstrated IgM anti-HEV antibodies. It was observed that none of these patients developed jaundice in the present epidemic, though they were consuming the same water and food and at least one member of their family was infected with hepatitis in the present epidemic.
Intra-familial spread
A history of jaundice developing simultaneously in other family members of the index patient living in the same area was seen in 44.1%. The clustering of jaundice patients was seen in a large number of families. There were 77 families that had 2 or more than 2 family members who were affected simultaneously i.e. there were multiple first cases in the same family. Of the 294 cases in the survey, 163 cases were the first case in their families. No family member presented later than 6 weeks following index case presentation.
Morbidity and Mortality
5 patients developed acute liver failure and died, of which 2 were pregnant women. The mortality rate in pregnant women was 5.1% (2/39). In the rest of the patients the mortality rate was 0.34% (3/859).
Coliform count and chlorine content
The major water supply to the town was from overhead 1 and four hand pumps. Water analysis and coliform counts of overhead 1 and the 4 hand pumps done on the 10th, 11th and 12thof February 2006 showed a coliform count of 30 mg/dL and traces of free chlorine. Overhead 2 and 4 and other hand pumps that were supplying water to other areas with distantly placed houses from the main town had low coliform counts. Only 32 persons residing in these areas and receiving water from overhead 2 suffered from hepatitis. Most of these people were labourers working in the main town and had consumed water from the affected area while on job duty.
What is new in this study?
1. nRT PCR may be used as a diagnostic tool to test for HEV during the early phase of infection in patients with negative IgM and IgG anti-HEV.
2. Exposure to HEV infection during the first epidemic (4 years back in the present study) produced lasting IgG anti-HEV antibodies and robably prevented further infection during this epidemic by producing rotective immune response against the virus.
3. In an epidemic setting if a patient has the classical clinical presentation of acute viral hepatitis and negative IgM anti-HEV but positive IgG anti-HEV it may still be diagnostic of acute hepatitis.
4. In healthy contacts of icteric patients with acute hepatitis E, IgM anti-HEV was seen in approximately two thirds of patients.
Discussion
The present study looked into the various serological and molecular markers of jaundice in patients with features suggestive of acute viral hepatitis and in their healthy contacts, during an epidemic in Khanpur, Rajasthan. At least 9 major epidemics of hepatitis E have been reported from India in the past. Although previous epidemics in India included a larger number of jaundiced patients, most of them lacked serological data. There were presumed causes in some cases[3,4,5] and in some epidemics the diagnosis was based only on the exclusion of hepatitis A and B.[6,8,9,19] There are only a few epidemics where the diagnosis of hepatitis E is based on specific serological tests.[7,20] In the present epidemic, the diagnosis of acute hepatitis E was based on clinical and biochemical parameters along with the presence of IgM anti-HEV or IgG anti-HEV and/or HEV RNA. There are only a few reports where all markers of HEV infection have been tested for to confirm the diagnosis of HEV infection. We determined the presence of anti-HEV (IgM and IgG) antibodies in patients whether they were in the first, second or third weeks of illness. There are infrequent studies (and none from India) where serology has been studied at different durations of illness during an epidemic.15
During previous hepatitis E epidemics, a higher frequency of overt clinical hepatitis has been documented amongst adults in comparison to children (below 15 years) and amongst men opposed to women.[14, 21] The present study also showed a similar trend in that a greater percentage of men (73.9%) and a lesser number of children (11.3%) were affected. We noted an overall attack rate of 4.5%. Earlier outbreaks reported variable attack rates lying between 1% and 15%.[23] The attack rate was closely related to the type of water consumed. In the present study people who received water from overhead 2 and were living outside the town had a lower attack rate (2.9%, 32/1080) when compared to people living in the area where water was supplied from overhead 1 (4.5, 827/18009). The coliform count and chlorine content also correlated well with the distribution of icteric hepatitis. As noted, the coliform count was higher and chlorine content lower in water supplied from overhead tank 1 as contrary to overhead tank 2 at the time when jaundice was first reported. These observations clearly point towards a faeces-contaminated water supply, probably leading to the hepatitis. There was a single peak in this epidemic and no individual developed hepatitis after six weeks of reporting of the final index case. This suggests that there was no person-to-person transmission, which is in accordance with a previous major epidemic report from India.[13] However there is still disagreement in the literature over person-to-person transmission of the HEV virus.
The present study revealed that the presence of IgM anti-HEV was highest in patients who were in their first week (80.2%) of illness and its level gradually fell in subsequent weeks (71.4% in the second week and 26.3% in the third week of illness). The level of IgG anti-HEV was low in patients in the first week (58.3%) and it increased in the second and third weeks of illness (77.1% in second week and 86% in third week). In another study, IgM anti-HEV was detected in 100%, 50% and 40% of patients after 1-40 days, 3-4 months and 6-12 months, respectively.[15] Other outbreaks suggest that IgM anti-HEV can be detected in more than 90% of patients within the first week to 2 months following the onset of hepatitis E.[16, 17] The variable sensitivity in detecting IgM anti-HEV in an outbreak was reported as 70-90% in symptomatic patients and 40-50% in asymptomatic patients depending on the kits that used different open reading frames and strains.[23} The lower level of IgM anti-HEV detected in our study may be because the kit we used comprised antigen from a strain different to the local strain. Mohanty et al[24] have shown that the sensitivity of commercially available kits to detect IgM anti-HEV (that detects peptides in the ORF2 and ORF3 regions) is lower than the kit (in house kits) which detected peptides of the ORF1, ORF2 and ORF3 regions. Hence, it was thought that if RdRp region of ORF1 was detected by RT PCR, it could detect the presence of HEV infection in patients, who had negative IgM or IgG anti-HEV, in the first week of their illness.
19 IgM and IgG anti-HEV negative blood samples from acute hepatitis patients during the first week were subjected to HEV RNA testing by RT-PCR using specific primers against the RdRp region of ORF1. In 16 out of 19 patients (84%) nRT PCR was positive in the first week. Various studies have shown the presence of HEV-RNA in acute hepatitis patients between 6 and 40 days following exposure to the HEV virus and a few days before the appearance of HEV antibodies.[25,26] Patients in whom RT-PCR was positive at day 6 had probably been picked up very early in the course of their illness. In an epidemic from Kanpur, RT-PCR was undertaken to detect HEV RNA in the stools of 10 patients and was positive in 6 patients. In yet another study on 116 patients in an epidemic in the Punjab, North India, 15 samples from patients in the first week of illness who were IgM anti-HEV positive were subjected to nRT-PCR assay, which was positive in 5 cases.[9] In all these studies nRT-PCR assay was used to detect the presence of
HEV-RNA in stool or blood samples, but not to confirm the causative agent. In the present study nRT-PCR was used as a diagnostic tool, in patients who tested negative for IgM and IgG anti-HEV. In yet another report the presence of the virus in blood or faeces (or both) was detected in 14 patients in whom IgM and IgG anti-HEV were not detected.[27] This shows the limitations of commercially available kits that show 70-90% sensitivity in outbreaks.[23] This may be the reason why only 84% of icteric patients tested positive for IgM anti-HEV in our study.
Unlike in other types of acute viral hepatitis, e.g. acute HBV infection where the level of IgG rises after IgM, we observed that both IgM and IgG appeared simultaneously in the first week. During the epidemic, clinically icteric patients, with elevated ALT, tested negative for IgM anti-HEV, but were positive for IgG anti-HEV, thus suggesting that the presence of only IgG antibodies may be diagnostic for acute hepatitis E in epidemic settings. In another study it was observed that IgG anti-HEV assay was more sensitive than IgM anti-HEV (86.7% vs. 53.3%) in detecting acute hepatitis E in the non-endemic setting.[28] However, in the present outbreak IgM anti-HEV assay proved more sensitive than IgG anti-HEV. In the town, there had been an epidemic of acute hepatitis E in 2002. Some of the patients who had then suffered from hepatitis E were traced and tested, and showed the presence of IgG anti-HEV in their blood, thus suggesting that IgG anti-HEV had persisted over four years. Earlier studies have also revealed that IgG can persist for 1 to 4.5 years after the acute phase of illness is over.[29,30] A long-term retrospective study from India showed that IgG anti-HEV may persist for up to 14 years following the population’s recovery from an epidemic.[31] An interesting and relevant observation in our study was that those persons who had suffered jaundice in the previous epidemic did not develop icterus in the present epidemic. Of the 173 subjects who suffered from jaundice in the previous epidemic, 93 were traced during the present epidemic. All 93 were positive for IgG anti-HEV and none suffered from jaundice and the transaminase level was also normal, all this inspite of the fact that they were consuming the same drinking water as their family members who did develop jaundice in the present epidemic; this possibly indicates that the presence of IgG anti-HEV protected these persons during this epidemic. A similar observation that IgG may be protective was made in another study and it was noted that not a single individual with acute hepatitis in the latter epidemics gave a history of hepatitis during the previous outbreaks.[29] This may help us develop a concept towards the making of an HEV vaccine using the indigenous strain prevalent in that locality. [29]
A high incidence of IgM anti-HEV was observed in healthy contacts (63%, 191/300) which indicates that during the epidemic a large proportion of contacts developed anicteric hepatitis E. This observation is similar to that seen in other epidemics.[1,2,3,4,5,6,7,8,9,10] HEV infection has been established as a major cause of mortality in pregnant women in India. In the present study 5.1% of jaundiced pregnant women died in comparison to 0.3% of non-pregnant individuals, they were each of them, in their third trimester and suffered from acute liver failure. The mortality in previous epidemics has been reported to be 12-20% [1,2,3,4,5,6,7,8,9,10]
We then conclude that, in an epidemic of acute hepatitis E the titres of IgM anti-HEV progressively diminish and those of IgG anti-HEV progressively increase during the course of the epidemic. IgG anti-HEV appears in the first week of illness and may be used for the diagnosis of acute hepatitis E in the clinical setting. IgG anti-HEV may be protective and may persist for a few years following infection. In areas with repeated episodes of acute hepatitis E outbreaks, patients who have suffered in a previous epidemic generally do not develop acute hepatitis E in subsequent epidemics. During the first week, in an epidemic setting, if a patient tests negative for IgM and IgG anti-HEV, nRT-PCR for HEV RNA can be used as a diagnostic tool to confirm the hepatitis. Thus detection of either IgG anti-HEV or HEV RNA may help in making a diagnosis of hepatitis E during an epidemic.
References
1. Aggarwal R, Krawczynski K. Hepatitis E: An overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15:9–20.
2. Krawczynski K, Aggarwal R, Kamil S. Hepatitis E. Infect Dis Clin North Am. 2000;14:669–87.
3. Dhamdhere MR, Nadkarni MG. Infectious hepatitis at Aurangabad. Report on an outbreak. Indian J Med Sci. 1962;16:1006–15.
4. Bhattacharji LM, Shah AL, Sampath Kumaran MA. Investigation of an outbreak of infectious hepatitis in a small town in West Bengal during July-October 1960. Ind J Med Res. 1963;51:550–61.
5. Patnayak S, Singh P, Pal SC, Koteswara Rao C, Shrivastav JB. An outbreak of infectious hepatitis in Siliguri, 1966. Indian J Med Res. 1968;56:1605–16.
6. Vishwanathan R. Infectious hepatitis in Delhi (1955-56). A critical study: Epidemiology. Indian J Med Res. 1957;45:1–30.a
7. Khuroo MS. Study of an epidemic of Non-A Non-B hepatitis. Possibility of another human hepatitis virus distinct from post transfusion Non-A Non-B type. Am J Med. 1980;68:818–24.
8. Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604.
9. Ray R, Aggarwal R, Salunke PN, Mehrotra NN, Talwar GP, Naik SR. Hepatitis E virus genome in stools of hepatitis patients during a large epidemic in North India. Lancet. 1991;338:783–4.
10. Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: Evidence for a non-A, non-B hepatitis virus etiology. Lancet. 1980;2:876–9.
11. Acharya SK, Panda SK, Saxena A, Gupta SD. Acute hepatic failure in India: A perspective from the East. J Gastroenterol Hepatol. 2000;15:473–9.
12. Skidmore SJ, Yarbough PO, Gabor KA, Reyes GR. Hepatitis E virus (HEV): The cause of waterborne hepatitis outbreak. J MedVirol. 1992;37:58–60
13. Aggarwal R, Naik SR. Hepatitis E. Interfamilial transmission versuswater born spread. J Hepatol. 1994;21:718–23
14. Panda SK, Acharya SK. Hepatitis E virus infection: where arewe? (Editorial). Natl Med J India. 1998;11:56–8.
15. Favorov MO, Khudyakov YE, Mast EE, Yashina TL, Shapiro CN, Khudyakova NS, et al. IgM and IgG responses antibodies to hepatitis E virus (HEV) detected by an enzyme immunoassay based on an HEV-specific artificial recombinant mosaic protein. J Med Virol. 1996;50:50–8.
16. Favorov MO, Fields HA, Purdy MA, Yashina TL, Aleksandrov AG, Alter MJ, et al. Serologic identification of hepatitis E virus infections in epidemic and endemic settings. J Med Virol. 1992;36:246–50.
17. Dawson GJ, Chau KH, Cabal CM, Yarbough PO, Reyes GR, Mushahwar IK. Solid-phase enzyme linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J Virol Methods. 1992;38:175–86.
18. Jameel S, Durgapal H, Habibullah CM, Khuroo MS, Panda SK. Enteric non-A, non-B hepatitis: epidemics, animal transmission and hepatitis E virus detection by the polymerase chain reaction. J Med Virol. 1992;37:263–70.
19. Sreenivasan MA, Banerjee K, Pandya PG, et al. Epidemiological investigations of an outbreak of infectious hepatitis in Ahemdabad city during 1975–76. Indian J Med Res. 1978;67:197–206
20. Kumar S, Ratho RK, Chawla YK, Chakraborti A. Virologicalinvestigation of hepatitis E epidemic in North India. Singapore Med J. 2006;47:769–73.
21. Mathur P, Arora NK, Panda SK, Kapoor SK, Jailkhani BL, Irshad M. Seroepidemiology of hepatitis E virus (HEV) in urban and rural children of North India. Indian Pediatr. 2001;38:461–75.
22. Acharya SK, Panda SK. Hepatitis E virus: epidemiology, diagnosis, pathology and prevention. Trop Gastroenterol. 2006;27:63–8
23. Myint KS, Endy TP, Gibbons RV, Laras K, Mammen MP Jr, Sedyaningsih ER, et al. Evaluation of diagnostic assays for hepatitis E virus in outbreak settings. J Clin Microbiol. 2006;44:1581–3.
24. Mohanty SK, Acharya SK, Dixit RK, Jha JK, Rai RR, Panda SK. IgM antibodies against hepatitis E virus (HEV) recombinant proteins and their importance in diagnosis of acute infection in endemic area. Ind J Gastroenterol. 2003;22:A5.
25. Tokita H, Harada H, Gotanda Y, Takahashi M, Nishizawa T, Okamoto H. Molecular and serological characterization of acute sporadic hepatitis E virus in 1993. J Gen Virol. 2003;84:421–7
26. Takahashi M, Kusakai S, Mizuo H, Suzuki K, Fujimura K, Masuko K, et al. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus is highly specific for diagnosis of acute HEV infection. J Clin Microbiol. 2005;43:49–56
27. Clayson ET, Myint KS, Snitbhan R, Vaughn DW, Innis BL, Chan L, et al. Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J Infect Dis. 1995;172:927–33.
28. Lin CC, Wu JC, Chang TT, Chang WY, Yu ML, Tam AW, et al. Diagnostic value of immunoglobulin G (IgG) and IgM anti hepatitis E virus (HEV) tests based on HEV RNA in an area where hepatitis E is not endemic. J Clin Microbiol. 2000;38:3915–8.
29. Chadha MS, Walimbe AM, Arankalle VA. Retrospective serological analysis of hepatitis E patients: a long-term follow-up study. J Viral Hepat. 1999;6:457–61.
30. Dawson JG, Mushawar IK, Chan KH, Gitnick GT. Detection of long lasting antibody to hepatitis E virus in US travelers to Pakistan. Lancet. 1994;340:426.
31. Khuroo MS, Kamili S, Dar MY, Moecklii R, Jameel S. Hepatitis E and long term antibody status [Letter]. Lan
|
|
|
 |
|
|