|
|
|
|
 |
 |
| |
 |
|
|
Quarterly Reviews |
|
|
|
|
|
Keywords :
|
|
|
Pankaj Tyagi, Kaushal Madan
Department of Gastroenterology,
GB Pant Hospital & Department of Medical Hepatology,
Institute of Liver and Biliary Sciences,
New Delhi
Corresponding Author:
Dr. Kaushal Madan
Email: k_madan_2000@yahoo.com
DOI:
http://dx.doi.org/
Abstract
It is clear that the major indication for the use of hematopoietic growth factors in hepatology is to counteract the adverse effects of interferons (neutropenia and thrombocytopenia) and ribavirin (hemolytic anaemia) during the treatment of hepatitis C infection. This is important because the probability of SVR depends on proper adherence to therapy (at least 80% of the requisite dose maintained for at least 80% of the requisite duration) and proper adherence can only be achieved if the side effects are reduced to a minimum. Even though the studies have demonstrated beyond doubt that the use of hematopoietic growth factors does indeed reduce the incidence and severity of these adverse effects and helps the patients to complete the course of therapy, the data on improvement of SVR is still limited. There is only one study of darbepoetin and filgrastim showing the beneficial effect on SVR. Even among the hematological side effects, possibly the only significant effect which limits the use of optimal HCV therapy is the hemolytic anaemia induced by ribavirin. The other two main side effects, i.e. neutropenia and thrombocytopenia are not clinically problematic. The use of such growth factors would be particularly effective if patients who have advanced liver disease or cirrhosis are able to receive adequate anti-viral therapy as has been demonstrated in the study of eltrombopag among HCV cirrhotics. Apart from this, other indications of G-CSF or GM-CSF use are still in the experimental stage. So, as of now, apart from erythropoietic factors, the role played by other hematopoietic growth factors in hepatology is limited. But future research, especially in the areas of immunotherapy of liver cancers and stem cell therapy for endstage liver disease, is surely going to give these factors their due place in hepatology.
|
48uep6bbphidvals|186 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Therepecific efficacy of haematopoitic growth factors (HGF) in the management of cancer and haematological problem have been well established. They have been predominantly used in the treatment of anaemia associated with chronic renal failure and chemotherapeutic drug-induced neutropenia. Recently, an orally active thrombopoietin receptor agonist has been introduced which can improve platelet levels in thrombocytopenic patients. There is also a growing area of use of these growth factors in hepatology, which mainly involves their use in the management of cytopenias induced during hepatitis C therapy. In addition, even though in the experimental stages, the immunomodulatory actions of some of these growth factors may be useful in some types of liver disease. But whether they have made a positive impact on disease outcome is to be seen. In the subsequent sections we shall discuss the roles of granulocyte colony stimulating factor (GCSF), erythropoietin (EPO) and eltrombopag in the management of liver diseases.
Granulocyte-colony stimulating factor (G-CSF)
The body’s major defense mechanisms against infectious agents include polymorphonuclear leucocytes, macrophages, natural killer cells, and cytotoxic lymphocytes. Upon activation, these cells can destroy and eliminate pathogenic microorganisms. G-CSF is a glycoprotein that plays an immunomodulatory role by affecting an increase in the leukocyte count, and upregulating phagocyte function during neutropenia. G-CSF is produced primarily by monocytes/macrophages, fibroblasts, and endothelial cells and acts on neutrophil precursors and mature neutrophils. It has been produced commercially by recombinant technology for clinical use in both glycosylated and non-glycosylated forms. Glycosylation stabilises the molecule by suppressing polymerisation and conformational change; this leads to resistance against degradation by human proteases. But both forms have similar biological activities.
The major action of G-CSF is to enhance the numbers and functions of neutrophils. G-CSF knockout mice develop chronic neutropenia with inability to control Listeria monocytogenes infection and failure to mobilise neutrophils during sepsis.1 This property of G-CSF is utilised in the treatment ofneutropenia due to drug-induced bone marrow suppression. G-CSF has also demonstrated the ability to mobilise CD34+hematopoietic stem cells from the bone marrow.[2] How this is used for the management of liver disease is discussed later.
G-CSF is considered a safe formulation and except for a 20%-30% incidence of musculoskeletal pain, it rarely produces life-threatening complications.
Thrombopoietic growth factors
Recombinant human interleukin 11 is a thrombopoietic growth factor that stimulates proliferation and maturation of megakaryocytes resulting in increased platelet counts. It has been used for the treatment of severe thrombocytopenia among patients receiving chemotherapy. It has also been used to treat HCV-related idiopathic thrombocytopenic purpura. In doses used by Fontana et al[3] in their study it also reduced the HCV RNA levels. But the major drawback of interleukin 11 isthe high cost and its propensity to cause fluid retention, which may lead to pedal oedema, pulmonary oedema and the capillary leak syndrome.
Thromobopoietin-receptor agonist (Eltrombopag) (GlaxoSmithKline) is a new, small-molecule, non-peptide, oral platelet growth factor. This drug interacts with the thrombopoietin receptor and induces proliferation and differentiation of megakaryocytes and results in an increase in platelet production. Eltrombopag therapy has been shown to stimulate megakaryocyte proliferation and differentiation and to cause a dose-dependent increase in platelet counts in both animal and human studies.[4,5]
Erythropoietin
Erythropoietin is a glycoprotein produced primarily in the kidneys that stimulates the division and differentiation of committed erythroid progenitors in the bone marrow. Recombinant human erythropoietin was first licensed as EPOGEN in 1989 for use in patients on renal dialysis, and it eliminated the need for repeated transfusions in this population. Recombinant erythropoietin is available commercially as Epogen, Eprex, and Procrit and as a hyperglycosylated longer-acting form, darbepoetin alfa. These drugs have been licensed for the treatment of anaemia
associated with chronic renal failure, chemotherapy for nonmyeloid cancers, zidovudine treatment of HIV, and to reduce the use of allogeneic blood transfusions in surgery patients. Epogen is indicated for the treatment of anaemia in dialysis patients with chronic renal failure.
Plasma erythropoietin levels remain relatively constant when haemoglobin levels remain at or above 12 g/dL but begin to rise rapidly and markedly when the haemoglobin level falls below 12 g/dL. It takes 3 to 4 days following erythropoietin stimulation for circulating reticulocytes to increase. Depending on the degree of anaemia, maturation of circulating reticulocytes into mature red blood cells (RBCs) may take an additional 1 to 3 days. Thus, any clinically significant increase in the haemoglobin levels is usually not observed in less than 2 weeks following erythropoietin stimulation and may require up to 6 weeks in some patients.
One significant adverse event reported with long- term use of erythropoietin in patients with chronic renal failure, is the development of pure red cell aplasia secondary to the development of anti-erythropoietin antibodies.[6] This may not be apparent on short-term use.
The burden of viral infection related chronic liver disease
Chronic HCV infection affects approximately 300 million people worldwide and is a major cause of cirrhosis, endstage
liver disease, and hepatocellular carcinoma (HCC).
Despite a decline in the incidence of acute HCV infection in recent years, the prevalence of HCV-related chronic liver disease is increasing in those who had acquired the infection before the introduction of the practice of screening of donor blood and blood products for HCV. Up to 80% of adults with HCV infection develop chronic viremia. However, only a quarter of chronically infected patients develop chronic hepatitis, a quarter of whom progress to cirrhosis. The disease in 1–4% of those who develop cirrhosis leads to liver cancer annually.[7]
In India the prevalence of anti-HCV in the general population is about 0.87% of which about 81% are viremic.[8] Based on these figures, more than 10 million Indians are estimated to be anti-HCV positive and >5-7 million are expected to be viremic. This is going to result in a large burden of HCV-related complications of cirrhosis and liver cancer in the coming years.
India is moderately endemic for the hepatitis B virus (HBV), with nearly 4% of its population, i.e. about 40 million people acting as chronic hepatitis B virus (HBV) carriers, most of who are asymptomatic (high endemicity >8%, intermediate 2%-8%, low <2%). Carriers of HBV are at increased risk of developing cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC). Although most carriers will not develop hepatic complications from chronic hepatitis B, 15% to 40% will develop serious complications during their lifetime. HBV is the major cause of chronic hepatitis, cirrhosis and primary liver cell cancer in India. About 50% of chronic liver disease in India is due to HBV infection and 20% due to HCV infection.[9]
HCV therapy: importance of adherence andcomplications of treatment
Despite many advances in research on HCV therapy, interferons and ribavirin are the mainstay of HCV treatment. Rapid improvement in the treatment of HCV infection during last decade includes, optimisation of duration of interferon according to prevalent genotype, increase in ribavirin dose and avaialbility of pegylated interferon. The protease inhibitors in the management of HCV infection are currently being evaluated. These therapies are associated with several hematopoietic side-effects such as anaemia, thrombocytopenia and neutropenia, causing interruptions in therapeutic schedule. Such interruptions or dose reductions are known to result in sub-optimal therapeutic efficacy.
There is no effective vaccination for HCV. The only option available for reducing the risk of cirrhosis and liver cancer in HCV infected patient is anti HCV treatment, is by means of effective anti-viral therapy. The best parameter for assessing response to therapy is the sustained virological response (SVR) and it has been demonstrated that patients with chronic hepatitis C who are able to achieve SVR have lower risk of progression to cirrhosis.[10] Patients who achieve SVR have a lower incidence of HCC as compared with those without SVR or those who were untreated.[11] In a study of 738 patients with chronic hepatitis C infection (594 received treatment and 144 did not), the hazard ratios for the development of HCC were 0.16 (0.04-0.62), 0.27 (0.09-0.79) and 0.74 (0.37-1.48) among sustained responders, transient responders and nonresponders respectively, signifying the importance of achieving SVR.[12]
Adherence to therapy is an important factor to achieve SVR. In a retrospective study comprising pooled data (1521 patients receiving either standard or pegylated interferon based regimens), it was demonstrated that patients receiving < 80% of one or both medications for < 80% of the duration of therapy had suboptimal SVR rates compared with patients who received > 80% of the assigned duration of therapy. Adherence to therapy (>80%) increased the SVR rate from 52% to 63% in patients who received pegylated interferon á 2b (PEG-IFN á2b) and Ribavirin (RBV) (p=0.04). In the groups receiving standard interferon the difference did not reach statistical significance and in the groups receiving weight- based ribavirin and pegylated interferon, the difference was significant only in genotype 1 patients.[13] In this particular study the authors were not able to dissect the importance of adherence to interferons or ribavirin. Even the advantage of achieving an early virological response (EVR, defined as a 2 log reduction in viral load at 12 weeks of therapy) is lost if adherence to therapy is not maintained for the remaining duration of treatmnet.[14]
The dose of ribavirin has been identified as an important variable for relapse. Patients who receive ribavirin in doses >10.6 mg/kg have higher SVR than those who receive <10.6mg/kg dose.[15] In a prospective randomised trial in 1311 HCV infected patients, it was demonstrated that the SVR was 63% and 52%, respectively in the groups receiving 1000-1200 mg/day and 800 mg/day doses of ribavirin along with pegylated interferon alpha 2a for 48 weeks.[16] This difference was apparent mainly in patients infected with genotype 1 HCV. Higher concentrations of ribavirin in whole blood after 4 weeks of treatment have also been associated with a higher response rate, and the probability of response increased with increasing concentration.17 Thus, it is apparent that in order to achieve an appreciable SVR, we need to maintain higher drug doses for at least 80% of the required duration of therapy. Because of a number of side-effects associated with the use of interferons and ribavirin, this goal can only be met if the associated side effects are aggressively managed or are not allowed to occur at all (if possible). The most important dose limiting side-effect associated with the use of interferons and ribavirin is hemolytic anaemia due to ribavirin, and bone marrow suppression, neutropenia and thrombocytopenia due to interferons.
Most of the available data on the importance of adherence to interferon therapy has been evaluated for hepatitis C treatment. Howevere, similar informations is not available for hepatitis B, but the importance of adequate treatment duration and dose of interferon should not be ignored in patients with hepatitis B as well.
Clinical use of hematopoietic growth factors
Anaemia
In hepatitis C patients, the National Anemia Action Council specifies the laboratory definition of anaemia as haemoglobin <11 g/dL or hematocrit <33%. Anaemia contributes to treatment-related fatigue, shortness of breath, and other symptoms that impair the quality of life (QOL).
Anaemia occurring during HCV therapy is multifactorial, which includes ribavirin induced hemolysis, interferon-induced bone marrow suppression and ribavirin induced downregulation of erythropoietin receptors.18 Ribavirin is concentrated in the red blood cells (RBCs) and ribavirin concentration in RBC is 60 times higher than that in the serum. Ribavirin is converted to ribavirin triphosphate in the RBC, leading to depletion of ATP within the RBCs causing oxidative membrane damage and hemolysis. Since RBCs lack mechanisms to hydrolyse ribavirin, it keeps getting accumulated. During combination antiviral therapy, haemoglobin usually decreases by 2.5–3.0 g/dL, within the first 4 weeks of treatment. Such decrease in haemoglobin is mantained through the treatment duration despite reduction in the dose of ribavirine. About 20% of patient receiving combination of interferon and ribavirin needs reduction in ribavirin dose for decrease in haemoglobin. [18,19] More than half of atients treated with interferon and ribavirin have been reported to have a haemoglobin decrease of 3 g/dL. [19] Anaemia contributes to treatment-related fatigue, shortness of breath, and other symptoms that impair the quality of life (QOL) further contributing to dose reduction/interruption of therapy in up to a third of patients.
The incidence of anaemia is significantly higher in certain subgroups of patients, such as HIV/HCV co-infected patients and patients who have recurrent HCV after liver transplantation.
Erythropoietin for treatment of anaemia during HCV therapy (Table 1)
Before the advent of erythropoietin physicians managed anaemia related to treatment of hepatitis C with reduction in ribavirin dose. The product information for peginterferon/ribavirin recommends ribavirin dose reduction by 200 mg/day (for peginterferon á-2b/ribavirin) or to 600 mg/day (for peginterferon á-2a/ribavirin) if the haemoglobin decreases to <10 g/dL in a patient without cardiac risk factors, and discontinuation of ribavirin if haemoglobin becomes <8.5 g/dL. Dose reduction generally stabilises the haemoglobin level but, on an average, produces an increase of only 1 g/dL.
Because of its effect on erythrocyte precursors, erythropoietin has been used to treat ribavirin-induced anaemia during the treatment of HCV. Dieterich and colleagues in an open label study randomised 36 patients to receive epoietin alfa ( 40,000 U/week) and 29 to receive placebo. Mean change from baseline haemoglobin level at week 16 was +2.8 g/dL for the epoietin alfa group and +0.4 g/dL in the placebo group; the difference was statistically significant (p<0.0001). The ribavirin dose reduction required was also significantly lower in the epoietin group (-34 mg/d vs. –146 mg/d; p=0.06)). In addition to a significantly higher haemoglobin level at week 16 (13.8 vs. 11.4; p<0.0001), HRQOL was also significantly better in patients receiving epoietin alfa at week 16.[20] In another double-blind study, 186 patients were randomised to receive epoietin alfa (n = 95) 40,000 U/week by subcutaneous injection for 16 weeks or placebo (n = 91) for 8 weeks. Epoietin alfa maintained the RBV dose in 88% of patients as compared with only 60% of patients on placebo. Patients on epoietin alfa in the double-blind part of the study demonstrated a mean increase in haemoglobin of 1 g/dL by week 4, with a further increase to 2.2 g/dL at the end of the study period. In the openlabel part of the study, patients on placebo crossing over to epoietin alfa achieved a mean increase in haemoglobin of 2.0 g/dL, and mean haemoglobin at the end of the open-label study was no different from that of patients maintained on epoietin alfa throughout the study.[21] Adverse events in this study were similar in both groups of patients except for nausea, which was more common in the epoietin alfa treated group. Six serious adverse events occurred during the study; five occurred in the epoietin group.
Although erythropoietin does reduce the incidence of anaemia and helps maintain adherence to anti-HCV therapy, its impact on the treatment of HCV will be demonstrated only if its use leads to an increase in the SVR. In a recent study of darbepoetin alpha (another erythropoiesis stimulating growth factor) and G-CSF for the management of anaemia and neutropenia during hepatitis C virus treatment, 101 patients were analysed. In addition to pegylated interferon alpha-2b and weight-based ribavirin dosing, they received darbepoetin alpha (3 mg/kg once every 2 weeks), if the haemoglobin was <10.5g/dL, and G-CSF if they had neutropenia. After 81 days, darbepoetin alpha produced an increase in the haemoglobin level by 1.9 + 1.0 g/dL%.22 Treatment with growth factors was an independent predictor for SVR. This is probably the only demonstration of an improvement in SVR with the use of erythropoeitic growth factors.
Neutropenia during HCV treatment
Neutropenia during HCV therapy occurs due to direct bone marrow suppression caused by interferon therapy. The absolute neutrophil counts decrease by 30-50% from the baseline value during HCV therapy with interferons.[23] The eutorophil counts usually drop in the first 2 weeks of therapy and then stabilise during the remainder of treatment. During trials using pegylated interferons, it was observed that neutropenia was the most common cause of dose reduction (being present in 18% of dose reduction cases) but rarely was responsible for discontinuation of therapy (<1% of discontinuation cases).
Neutropenia during HCV treatment
Neutropenia during HCV therapy occurs due to direct bone marrow suppression caused by interferon therapy. The absolute neutrophil counts decrease by 30-50% from the baseline value during HCV therapy with interferons.[23] The neutorophil counts usually drop in the first 2 weeks of therapy and then stabilise during the remainder of treatment. During trials using pegylated interferons, it was observed that neutropenia was the most common cause of dose reduction (being present in 18% of dose reduction cases) but rarely was responsible for discontinuation of therapy (<1% of discontinuation cases).
The fear of infectious complications with interferon induced neutropenia has led to recommendations (by the manufacturers of interferons) that pegylated interferon dose should be reduced by 25-50 % if the neutrophil count goes below 750/mm3 and the drug should be discontinued if the neutrophil count goes below 500/mm3. However such an increase in infectious complications with interferon therapy has not been demonstrated in clinical studies (discussed later).
G-CSF for treatment of neutropenia during HCV therapy
Although there are no guidelines for the administration of GCSF in the treatment of neutropenia during HCV therapy, many hepatologists use this growth factor whenever there is a fall in neutrophil count during interferon use. In a recent survey conducted in France on the practice of physicians prescribing growth factors during HCV therapy, it was shown that G-CSF was prescribed in a dose of 300 ìg, 1-3 times a week. The main indication was a neutrophil count of 400-750/mm3. The independent factors for prescribing G-CSF for HCV treatment were younger age of the physician (<45 years), practice in a university hospital and high load of HCV patients.[24]
The issue of G-CSF making an impact on HCV therapy has two aspects. First, has the use of G-CSF resulted in significant improvements in the SVR? Again, there is only one study which has tried to answer this. [22] In this study, both darbepoetin and G-CSF were used as hematopoietic growth factors along with pegylated interferon alpha 2b and weightbased ribavirin therapy. Patients who developed neutropenia (absolute neutrophil counts <750/mm3) received G-CSF in a dose of 150 ì gm/ every week to 300 ì gm thrice a week. Low viral load, non-genotype 1 infection and treatment with hematopoietic growth factors were independently associated with SVR.
Second, do we really need to use G-CSF for low neutrophil counts when studies have not been able to demonstrate any increase in infectious complications secondary to interferon induced fall in white blood cell counts? There are no reports to implicate neutropenia as a risk factor for infections during anti-HCV therapy. In a retrospective study comprising, 192 chronic hepatitis C patients, 67 infectious complications occurred in 57 patients, but the infectious complications were neither correlated with the nadir of the neutrophil counts nor with the degree of fall in neutrophil counts from baseline.[25] In another study of 119 patients who received standard interferon and ribavirin therapy, the neutrophil count decreased to <750/mm3 in 9% and to <500/mm3 in 2%. it was demonstrated that 9% developed neutrophil counts <750/mm3 and 2% developed neutrophil counts <500/mm3. Even though 18% patients developed infectious complications, a comparison of patients with and without infection revealed no difference in either baseline neutrophil counts nor in the degree of fall in neutrophil counts during therapy.[26] Therefore, evidence in favour of association between neutropenia during HCV treatment and infection is not available. Further, randomized trials need to be carried out evaluating the need and impact of G-CSF as an adjuvant / add-on with interferon-based regimens for HCV.
Having said that, it is also true that whenever interferon based treatment is given to patients who are cirrhotic[27,28] or who have HIV co-infection or in post-liver transplantation cases, the incidence of infectious complications increases. In such situations the use of G-CSF may be justified in order to maintain adequate neutrophil counts. But again systematic studies need to be carried out even in such situations.
Thrombocytopenia during HCV therapy
Thrombocytopenia is a common manifestation of chronic liver disease, and its prevalence correlates with the severity of hepatic injury. In chronic liver disease, it is usually a manifestation of portal hypertension and hypersplenism. In addition hepatitis C may cause thrombocytopenia by autoimmune mechanisms. Patients receiving interferon or pegylated interferon in chronic hepatitis experience a fall in platelet count, due to bone marrow suppression, but platelet counts < 75,000/mm3 are rare and seldom require dose reduction or discontinuation. Peck-Radosavljevic et al,29 showed in their study of cirrhotic and non-cirrhotic HCV patients on standard IFN, that platelet counts decreased by 32% and 35%, respectively. They also demonstrated a blunted thrombopoietin response to falling platelet levels among patients with advanced liver disease (cirrhotics vs. noncirrhotics), suggesting that recombinant human thrombopoietin may be of value in patients developing thrombocytopenia during interferon therapy.
The manufacturers of pegylated interferon suggest that the dose of PEGIFNa-2a should be reduced by 50% in patients who develop a platelet count of <50,000/mm3, and should be discontinued in patients who develop a platelet count of <25,000/mm3. Reduction by 50% is recommended with PEGIFNa-2b in patients who have a platelet count of <75,000/mm3, and discontinuation is recommended in patients who have a platelet count of <50,000/mm3. Even though thrombocytopenia occurs during therapy with interferons, spontaneous bleeding episodes rarely occur in such patients.
Thrombopoietic growth factors for treatment of thrombocytopenia during HCV therapy[3]
Recombinant human interleukin-11 (Neumega [oprelvekin]) As mentioned earlier, one study has used interlueukin 11 to treat HCV-related thrombocytopenia, but no published study has looked for the effect of interleukin 11 on thrombocytopenia induced by interferon therapy.
Thromobopoietin-receptor agonist (Eltrombopag)
In a randomised controlled trial, eltrombopag was used in doses of 30 mg, 50 mg and 75 mg among patients with idiopathic thrombocytopenic purpura who were resistant to standard therapy. Twenty eight percent, 70% and 81% of these patients in the three dose groups, respectively were able to achieve an increase in platelet count to over 50000/mm3. [30] Availability of an orally active drug for management of thoromobocytopenia, makes management very easy, especially because it avoids the dangers and inconvenience of repeated platelet transfusions. In patients of HCV-related cirrhosis undergoing antiviral therapy, ongoing administration of eltrombopag at 75 mg daily enabled significantly more patients to both commence combination antiviral therapy with pegylated IFN and ribavirin and then to successfully complete 12 weeks of treatment compared with placebo (65% vs. 6%). Importantly, despite eltrombopag therapy, platelet counts decreased in all eltrombopag treatment groups while patients received antiviral therapy, but continued to remain over 50,000/mL3. Insignificant side effects such as headache, dry mouth, abdominal pain and nausea have been reported with the use of eltrombopag.[31] But it is yet to be seen whether this completion of therapy with concomitant use of eltrombopag will translate to improvement in the SVR in patients of HCV undergoing therapy with interferon and ribavirin. If the patients who have advanced fibrosis or cirrhosis receive adequate therapy, and are able to eradicate the virus from their bodies, they will have a lower risk of developing complications, and this group will then constitute the best target for the use of such growth factors. This is the only way eltrombopag will have an impact on HCV treatment.
Other indications of hematopoietic growth factors in hepatology
G-CSF for mobilisation of bone marrow stem cells
G-CSF also promotes the proliferation and mobilisation of bone marrow progenitor cells [peripheral blood stem cells (PBSC)], which may be pluripotent and may differentiate in to cells of hepatic lineage helping in liver regeneration. Four to six days after administration of G-CSF CD34+ PBCs begin to rise in the peripheral blood.[32] The optimal dose of G-CSF for this purpose is about 10-16 ì g/kg/day leading to a harvest of about 4.9 x 106/kg to 7.1 x 106/kg PBSCs on day 5.[33]
In a recent study G-CSF was used to help mobilise autologous bone marrow derived stem cells (CD 34+) from patients with alcoholic liver disease. The cells after leukapharesis and in-vitro expansion were transfused back in to the hepatic artery of these patients resulting in improvement in bilirubin, Child’s scores and ascites.[34] Although stem cell therapy for chronic liver disease is still in the experimental stages, the use of G-CSF is integral to this form of therapy and in the near future, if this therapy succeeds in improving the survival of patients with end-stage liver disease, G-CSF will have a significant impact in the field of hepatology.
G-CSF/Granulocyte Macrophage (GM)-CSF as immunotherapeutic agents for liver tumours
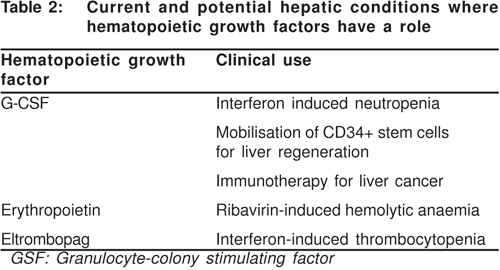
GM-CSF has the ability to induce dendritic cells, which in turn stimulate tumour specific cytotoxic T lymphocytes (CTLs) leading to tumour regression.[35] GM-CSF has been used in experimental liver tumours along with IL-12 as a form of successful cancer immunotherapy.[36] But this too is experimental use of GM-CSF and a lot of research needs to done before this will make any difference in the practice of hepatology. Table 2 summarises the beneficial effects of hematopoieitic growth factors in liver disease.
GM-CSF as an immunoadjuvant along with hepatitis B vaccine
Although the response rate (development of protective anti-HBs titres) to the recombinant hepatitis B vaccine in healthy individuals is more than 95%, lack of response is an important problem among patients with chronic renal failure on hemodialysis. Ensuring seroconversion is also important in individuals who are at high risk of acquiring HBV infection, such as healthcare professionals, laboratory workers, and close contacts of infected patients. In such cases GM-CSF has been used as an adjuvant in order to increase the efficacy of the recombinant hepatitis B vaccine. The first study from India compared the efficacy of HBV vaccination at 0, 1, 2 and 6 months with or without a single dose of GM-CSF (3 ì g/kg) on day 1, among patients with renal failure. 100% patients who received GM-CSF demonstrated seroconversion compared to 44% in the other group.[37] Subsequently there have been meta-analyses which have demonstrated that GM-CSF given as an adjuvant along with the hepatitis B vaccine, improves seroconversion rates, with pooled OR ranging from 1.54 to 4.6.[38,39] But the major impact of this practice will mainly be among patients with chronic renal failure on hemodialysis.

References
1. Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, et al. Mice lacking granulocyte colony stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency and impaired neutrophil mobilization. Blood. 1994;84:1737-46.
2. Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim9r-metHuG-CSF): the first ten years. Blood. 1996;88:1907–29.
3. Fontana V, Dudkiewicz P, Jy W, Horstman L, Ahn YS. Interleukin-11 for treatment of hepatitis C associated ITP. Act Haematol. 2008;119:126–32.
4. Sellers T, Hart T, Semakin M, Murthyl K. Pharmacology and safety of SB–497115–GR, an orally active small molecular weight TPO receptor agonist in chimpanzees, rats and dogs. Blood. 2004;104:2063A.
5. Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase I clinical study of eltrombopag, an oral, non-peptide thrombopoeitin receptor agonist. Blood. 2007;109:4739–41.
6. Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, et al. Pure red cell aplasia and epoietin therapy. N Eng J Med. 2004;351:1403–8.
7. Alter HJ, Steeff LB. Recovery, persistence and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35.
8. Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Maity SG, Naik TN, et al. Hepatitis C virus infection in the general population: A community-based study in West Bengal, India. Hepatology. 2003;37:802–9.
9. Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India. 2006;19:203-17.
10. Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13.
11. Yu ML, Lin SM, Chuang WL, Dai CY, Wang JH, Lu SN, et al. A sustained virological response to interferon or interferon/ribavirin reduces hepatocellular carcinoma and improves survival in chronic hepatitis C: a nationwide, multicenter study in Taiwan. Antivir Ther. 2006;11:985–94.
12. Tanaka H, Tsukuma H, Kasahara A, Hayashi N, Yoshihara H, Masuzawa M, et al. Effect of interferon therapy on the incidence of hepatocellular carcinoma and mortality of patients with chronic hepatitis C: a retrospective cohort study of 738 patients. Int J Cancer. 2000;87:741–9. Int J Cancer. 2000;87:741–9.
13. Mc Hutchison JG, Manns M, Patel K, Pyonard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype 1 infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–9.
14. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Eng J Med. 2002;347:975–82.
15. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958–65.
16. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon alpha 2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55.
17. Jen JF, Glue P, Gupta S, Zambas D, Hajian G. Population pharmakokinetic and pharmacodynamic analysis of ribavirin in patients with chronic epatitis C. Ther Drug Monit. 2000;22:555–65.
18. Canonico PG, Kastello MD, Spears CT, Brown JR, Jackson EA, Jenkins DE. Effects of ribavirin on red blood cells. Toxicol Appl Pharmacol. 1984;74:155–62.
19. Sulkowski MS, Wasserman R, Brooks L, Ball L, Gish R. Changes in heamoglobin during interferon alpha 2b plus ribavirin combination therapy for chronic hepatitis C virus infection. J Viral Hepat. 2004;11:243–50.
20. Dieterich DT, Wasserman R, Brau N, Hassanein TI, Bini EJ, Bowers PJ, et al. Once weekly Epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491–9.
21. Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS, et al. Epoetin alfa maintains ribavirin dose in HCV infected patients: a prospective double blind, randomized controlled study. Gastroenterology. 2004;126:1302–11.
22. Younossi ZM, Nader FH, Bai C, Sjogren R, Ong JP, Collantes R, et al. A phase II dose finding study of darbepoetin alpha and filgrastim for the management of anemia and neutropenia in chronic hepatitis C treatment. Viral Hepat. 2008;15:370–8.
23. Wong S, Kaita K, Gauthieer T, Jones S, Minuk GY. A comparative trial of recombinant interferon alpha 2a versus alpha 2b on myelosuppression in healthy adult volunteers. Hepatogastroenterology. 1996;43:301–5.
24. Thévenot T, Cadranel JF, Di Martino V, Pariente A, Causse X, Renou C, et al. A national French survey on the use of growth factors as adjuvant treatment of chronic hepatitis C. Hepatology. 2007;45:377–83.
25. Cooper CL, AL-Bedwawi S, Lee C, Garber G. Rate of infectious complications during interferon-based therapy for hepattis C is not related to neutropenia. Clin Infect Dis. 2006;42:1674–8.
26. Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, et al. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology. 2002;36:1273–9.
27. Kumar R, Kumar S, Sharma BC, Singh J, Sarin SK. Antiviral therapy in advanced chronic liver disease due to hepatitis C virus infection: pilot study. J Gastroenterol Hepatol. 2005;20:527–35.
28. Iacobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, et al. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: A controlled study. J Hepatol. 2007;46:206–12.
29. Peck-Radosavljevic M, Wichlas M, Pidlich J, Sims P, Meng G, Zacherl J, et al. Blunted thrombopoietin response to interferon alfa-induced thrombocytopenia during treatment for hepatitis C. Hepatology. 1998;28:1424–9.
30. Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237–47.
31. Mc Hutchison JG, Dusheiko G, Shiffman ML, Rrodriguez-Torres M, Sigal S, Bourliere M, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–36.
32. Sheridan WP, Begley CG, Juttner CA, Szer J, To LB, Maher D, et al. Effect of peripheral blood progenitor cells mobilized by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–4.
33. Kroger N, Renges H, Sonnenberg S, Kruger W, Gutensohn K, Dielschneider T, et al. Stem cell mobilization with 16 mg/kg vs 10 mg/kg of G-CSF for allogenic transplantation in healthy donors. Bone Marrow Transplant. 2002;29:727–30.
34. Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, et al. Autologous infusion of expanded mobilized adult bone marrow derived CD-34+ cells in to patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–8.
35. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates poten, specific and long lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539-43.
36. Chang CJ, Chen YJ, Huang KW, Cheng HW, Chan HF, Tai KF. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology. 2007;45:746–54.
37. Kapoor D, Aggarwal SR, Singh NP, Thakur V, Sarin SK. Granulocyte-macrophage colony stimulating factor anhnaces the efficacy of hepatitis B virus vaccine in previously unvaccinated haemodialysis patients. J Viral Hepat. 1999;6:405–9.
38. Cruciani M, Mengoli C, Serpelloni G, Mazzi R, Bosco O, Malena M. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination: a meta-analysis. Vaccine. 2007;25:709–18.
39. Fabrizi F, Ganeshan SV, Dixit V, Martin P. Meta-analysis: the adjuvant role of granulocyte macrophage-colony stimulating factor on immunological response to hepatitis B virus vaccine in end stage renal disease. Aliment Pharmacol Ther. 2006;24:789–96.
40. Talal AH, Weisz K, Hau T, Kreiswirth S, Dieterich DT. A preliminary study of erythropoietin for anemia associated with ribavirin and interferon-alpha. Am J Gastroenterol. 2001;96:2802– 4.
41. Gergely AE, Lafarge P, Fouchard-Hubert I, et al. Lunel-Fabiani F. Treatment of ribavirin/interferon-induced anemia with erythropoietin in patients with hepatitis C. Hepatology. 2002;35:1281–2.
|
|
|
 |
|
|