48uep6bbphidvals|245
48uep6bbphidcol2|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
In the last one and a half decades, the concept of free radical mediated oxidative stress (OS) has gained tremendous scientific momentum right from studying its role in the pathophysiology of disease to its therapeutic implications. Almost all gastrointestinal (GI) diseases have been evaluated for underlying OS as a causative factor and intervention with antioxidants attempted, both dietary as well as synthetic. However, the results in these studies have been varied and require epidemiological and clinical research before the findings can be applied universally and intervention with antioxidants endorsed. This article focuses on alcoholic and non-alcoholic liver diseases, pancreatitis and inflammatory bowel disease.
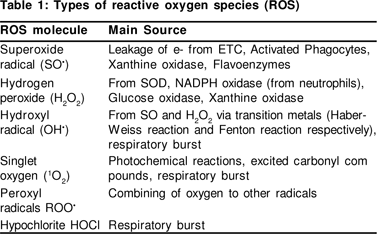
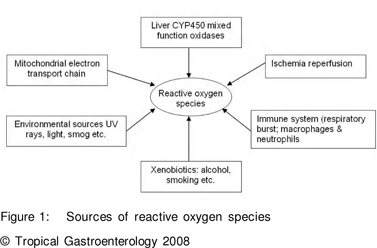
OS has been defined as an imbalance in the activity of pro-oxidants and antioxidants in favour of the former. Pro-oxidants favour free radical (FR) formation whilst antioxidants inhibit or retard the same. Reactive oxygen species may include various radicals and non-radicals (Table 1). Besides well known exogenous sources of FR, normal physiological processes in the body also produce FR and these are very essential for normal biochemical processes and are widely distributed. The sources of FR are listed in Figure 1.
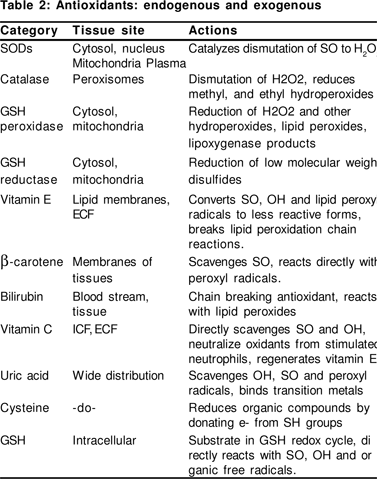
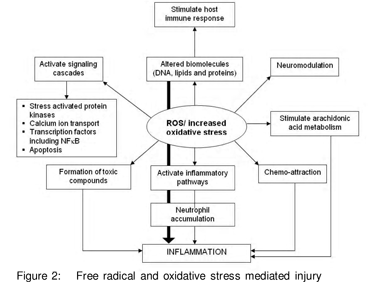
The body has an entire gamut of antioxidant defenses, including endogenous antioxidants [enymatic such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and non-enzymatic antioxidants such as glutathione (GSH), uric acid, melatonin, ferritin, ceruloplasmin etc] as well as exogenous antioxidants [that are mainly diet derived including vitamins A, C, E, carotenoids, phenolic compounds, various other plant pigments and some metal ions such as selenium and zinc] (Table 2). The OS injury proceeds via four critical steps; membrane lipid peroxidation, protein oxidation, DNA damage and disturbance in reducing equivalents of the cell. This leads to cell destruction, altered signaling pathways and genetic alteration (Figure 2).
The GI tract has a particularly important part to play in the generation and damage through free radicals. The GI tract is bombarded with diet accompanying FR and pro-oxidants and various xenobiotics that are metabolised mainly in the liver and smaller quantities at other sites of the GI tract. It has been postulated that the GI tract processes more than 25 tons of food in an average life time, which represents the largest load of antigens and xenobiotics confronting the human body![1]
Xenobiotics are metabolised in the body via the phase I and the phase II pathways, where in phase I, CYP450 enzymes add a reactive group to the xenobiotic compound, which produces reactive molecules that may be more toxic than the parent molecule. The phase II conjugates it with biomolecules and helps in excretion of these compounds. When there is an increased xenobiotic load, the phase I and /or phase II enzymes involved in detoxifying can be induced, which may result in unmatched activity of the two phases resulting in leakage of the reactive intermediates. Therefore, clinically the most important function of CYP2E1 is not only ethanol oxidation, but also its extraordinary capacity to convert many xenobiotics to highly toxic compounds, which may cause a redox balance disturbance.[2]
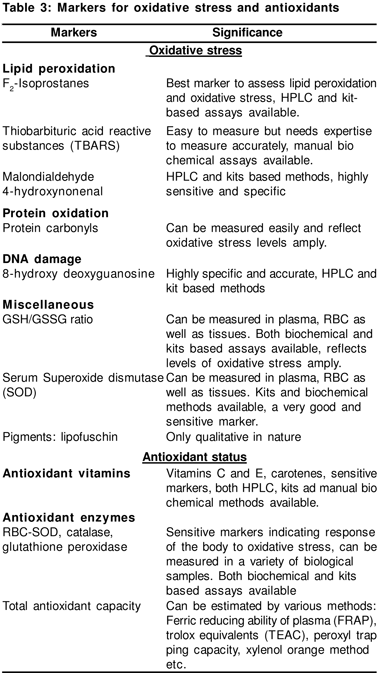
Redox regulation mediates numerous cellular responses and contributes to several physiological diseases. The transcription factor nuclear factor kB (NFkB) is known as redox sensitive and responds to OS. It plays a central role in immune responses and inflammation (via cyokines) through regulation of gene expression of cytokines and other immune response genes.[3] OS can stimulate cytokines and cytokines can induce OS,;an interplay of the two. There are several markers of OS and antioxidants that may be measured in various body tissues and fluids (Table 3).
Oxidative stress and antioxidants in liver diseass
Alcoholic liver diseases
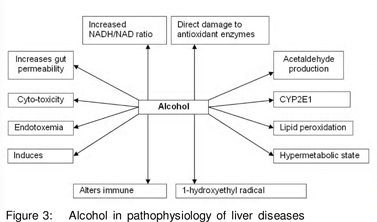
Alcohol remains the main contributory and most studied factor in liver disease. Several theories have been given to explain the damage caused by alcohol and OS remains a major one (Figure 3).
The role of alcohol in liver diseases has been evidenced by lipid peroxidation products (LPO)[4] including 8- isoprostanes,[5] malondialdehyde (MDA).[6] The adducts of LPO with other biomolecules may also be formed in ALD[7] and invoke inflammatory and immune responses.[8] Levels of SOD have been reported to increase9 as well as decrease[10] in patients with ALD. OS can also stimulate fibrosis by stellate cell activation and 4-hydroxynonenal (HNE) has a pro-fibrotic stimulus.[11] We conducted a study in patients with ALD and observed an increased OS amongst 45 patients with ALD as indicated by increased LPO (estimated as thiobarbituric acid reactive substances; TBARS) and serum SOD levels. However, the total antioxidant capacity (TAC) as measured by the ferric reducing ability of plasma (FRAP) and plasma vitamin C were not different between the cases and controls. The duration and amount of alcohol consumption correlated with the OS levels. This suggests that alcohol is the main contributor towards the pathogenesis of ALD.[12]
Liver is the most important source of GSH in the blood and Sadenosyl methionine (SAMe) is the key methyl donor in GSH synthesis. Patients with ALD have been shown to have deficiency of SAMe synthase.[13] Thus, SAMe supplementation may restore the cellular GSH levels. In a 24-month RCT with 123 alcoholic cirrhosis patients, SAMe supplementation improved survival and delayed the need for liver transplantation.[14] Oral administration of SAMe for 6 months significantly increased hepatic glutathione concentration in ALD patients.[15,16] A recent randomised controlled trial (RCT) with antioxidants in patients with ALD using N-acetyl cysteine (NAC), vitamins A and E, biotin, selenium, zinc, manganese, copper, magnesium, folic acid and coenzyme Q, was unable to show any effect on 6 month survival in severe alcoholic hepatitis.[17] Vitamin E has been shown to exert its beneficial effects by stabilising membranes, quenching lipid peroxide radicals, reducing NFkB activation and TNF-a production.
However, it has not shown any clinical benefit in two trials. [19 ,20]
Non-alcoholic fatty liver diseases
NAFLD has become common of late and is histologically similar to alcoholic hepatitis. It is commonly diagnosed in patients with obesity, diabetes mellitus, hyperlipidemia and other metabolic syndromes. It has been suggested that in NAFLD, baseline steatosis requires a second hit capable of inducing inflammation, necrosis and fibrosis.[21] Endotoxin induced cytokine release and OS induced lipid peroxidation,[22] as well as mitochondrial dysfunction appear to be key factors in NASH as they lead to alteration in mitochondrial electron transport chain, generating more ROS.[23]
Recently, high levels of circulating antibodies against LPO have been demonstrated among patients with NAFLD.[24] We had also earlier reported an increased OS in patients with NAFLD.[25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] TBARS was significantly higher in patients with NAFLD as compared to healthy controls. But FRAP was higher in the NAFLD group, which was surprising. This may be attributed to the high uric acid levels in these patients, which is a common finding in this condition.
Further, biochemical and histological improvement has been demonstrated in patients with NAFLD who have been treated with antioxidants.[26 ,27 ]Vitamin E has been tested in clinical trials in NAFLD and has been shown to have a beneficial effect in adults[28] and children.[29] However, in a study by Kugelmas et al, vitamin E was not effective in treatment of NASH.[30]
Viral liver diseases
Different mechanisms have been suggested to explain the pathophysiology of chronic viral hepatitis and include immunological damage to the liver, cytotoxicity of viral products and OS.[31] By their role in cell activation, ROS may facilitate or even promote replication of these parasites depending on the cell and virus involved.[32] Virus-induced activation of phagocytes releases not only ROS but also pro-oxidant cytokines, such as TNF and interleukin-1 (IL-1).[33] Studies have reported that ferritin, iron levels and MDA are significantly increased in patients with HCV when compared to HBV patients and healthy controls.[34 ,35] HCV patients have increased LPO products and adducts, protein adducts and damaged DNA[36 ,37] along with elevated SOD activity.[38]
Alpha-tocopherol supplementation along with fermented papaya extract showed an improvement in the markers of OS and antioxidant status including 8-hydroxy-deoxyguanosine (8-OH dG) as well as serum alanine transferase (ALT).[39] In another RCT, supplementation with vitamins C and E and Se amongst patients with hepatitis C showed increase in vitamin E, C and erythrocyte glutathione peroxidase levels, however, the viral load was not altered.[40] In CHB, vitamin E supplementation resulted in significant normalisation and significant negativization of HBV-DNA along with an increase in vitamin E levels.[41] High doses of a-tocopherol increased vitamin E, decreased ALT, AST and treatment cessation again increased ALT and AST.[42] While these studies have shown benefit with antioxidants like vitamin E others have excluded the beneficial effect of vitamin E in combination with ribavirin,[43] NAC[44] and selenium in viral liver diseases.[45]
Acute liver failure
Acute liver failure (ALF) is characterised by sudden and massive necrosis and apoptosis of hepatocytes. The main causes of ALF are viral hepatitis[46] or drug induced liver toxicity,[47] involving the CYP enzymes as well as genetic susceptibility. Further, the dying hepatocytes can be a source of massive OS along with a depletion of antioxidant defenses. OS can contribute to further hepatocyte loss, and impede regeneration, culminating in a vicious cycle. We studied OS and antioxidant profile on 3 days in a 7-day period in patients with acute hepatic failure.[25] We observed that TBARS was significantly higher in patients as compared to that of controls and showed a significant decline from baseline towards day 7. Serum SOD was also significantly raised at baseline in patients and decreased towards day 7 but was not significant. Also the FRAP was lower in patients when compared with healthy controls on all days.
Pancreatic diseases
Acute pancreatitis
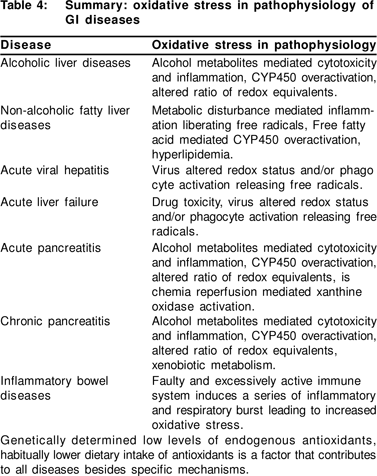
The aetiology of acute pancreatitis (AP) may be alcohol, biliary tract disease, tumours, viral infections, drugs, hyperparathyroidism, hyperlipidemia or trauma. The systemic complications of AP are similar to those seen in the systemic inflammatory response syndrome (SIRS) or multiple organ dysfunction syndrome (MODS). In human AP, increased amounts of LPO [48,49,50] and subnormal to unaltered levels of antioxidant vitamins in the blood have been reported.[50,51,52] In general antioxidant levels were lower in these patients.[53]
Antioxidant supplementation studies have hitherto been unable to establish the efficacy of therapy. Till date there are three published clinical trials in this regard. In the first trial 46 patients with AP were given supplements of selenium, N-acetyl cysteine, ascorbic acid, b-carotene and a-tocopherol. Supplementation restored vitamin C and selenium levels however, no effect was observed on morbidity and mortality.[54]
The second trial attempted supplementation with b-carotene, 12-h prior to endoscopic retrograde cholangiopancreatography (ERCP), which is known to initiate AP. But in this trial, single dose of b-carotene was not protective.[55] Recently an RCT was undertaken that included 43 patients with AP, 22 in the antioxidant arm and 21 in the placebo arm. The patients were given supplements of NAC, selenium and vitamin C, markers of OS decreased and antioxidant levels increased (ascorbic acid, selenium, GSH/GSG ratio and C-reactive protein) however, no effect was observed on clinical outcome (organ dysfunction, length of hospital stay and mortality).[56] We conducted a single blind RCT at our Institute including 53 patients, 30 randomised to standard medical treatment and 23 patients to placebo and antioxidants (vitamin C 700 mg, NAC 600 mg, vitamin E 75 IU, b-carotene 3000 mg, vitamin A 15,000 IU daily). Following supplementation, there was a decrease in the OS (TBARS and serum SOD) and an increase in the markers of antioxidant status (vitamin C and FRAP). However, no clinical benefit was observed, which may have been due to the small sample size.[57]
Chronic pancreatitis
Chronic pancreatitis (CP) is a progressive inflammatory disease of the pancreas, characterised by chronicinflammation, slow destruction of pancreatic parenchyma and progressive fibrosis.[58] Pain is the major problem in 90% of the patients with CP accompanied with maldigestion and diabetes in later stages.[59] Role of oxidative stress and antioxidants in the pathophysiology of CP has been suggested in many studies.[60 ,61 ,62]
The OS in acinar cells may result from CYP induction, concurrent exposure to a chemical that undergoes bioactivation and insufficiency of micronutrients that are required to sustain GSH stores (Figure 4). CP involves uncoordinated detoxification reactions, loss of glutathione in the acinar cells and compromised methionine trans-sulphuration pathway responsible for the synthesis of GSH. Free radical peroxidation products (FROPs) may act as second messengers and block exocytosis, leading to increased autophagy and crinophagy, thus diverting pancreatic enzymes into the interstitium, and degranulation of mast cells, resulting in inflammation mediated by chemotaxis, and hence pain.[63]
We have earlier reported a poor dietary antioxidant intake in patients with CP as compared to that of healthy controls.[64] We have also shown increased OS and low levels of antioxidants in patients with CP.[65] Uden et al[66] performed a 20-week double blind placebo controlled cross-over trial, including 20 patients, and showed that 6 patients on placebo had attacks compared with none on active treatment. A recent randomised, double blind, placebo-controlled cross-over trial evaluated the efficacy of combined antioxidant supplementation in patients with chronic pancreatitis.[67] The study included 36 patients and antioxidants were given for 10 weeks. Final data was from 19 patients who completed the 20-week trial. It was observed that there was significant improvement in the quality of life and reduction in pain. A pilot study at our centre also showed a decreased OS and improved antioxidant status in patients with CP following antioxidant supplementation. This study also showed clinical benefit in terms of pain relief.[68]
Inflammatory bowel disease
Inflammatory bowel disease (IBD), both Crohn’s disease (CD) and ulcerative colitis (UC) are characterised by chronic or relapsing inflammation. The uncontrolled and sustained host immune response is coupled with extensive inflammatory burst in the intestine with the presence of inflammatory cells and OS markers.[69] Colonic biopsy showed an increased OS in patients with IBD and correlated with disease activity.[70] The main sites of ROS production in IBD are phagocytic leukocytes. During episodes of inflammation, these cells exhibit massive infiltration of the intestinal mucosa and liberate large amounts of ROS. Several studies have shown poor antioxidant status in these patients such as low TAC,[71] increased SOD,[72] antioxidant vitamins[73 ,74 ,75] and glutathione.[76]
In a randomised controlled trial, 57 patients with stable CD were given vitamin E and vitamin C supplements for 4 weeks; the OS decreased and antioxidant levels improved and the disease activity remained stable.[77] In another RCT, it was observed that fish oil, b-carotene, selenium, vitamin E and vitamin C decreased the OS but no effect on disease activity was observed.[78] Geerling et al[79] gave 25 CD patients in remission selenium, b-carotene, vitamin E and vitamin C supplements which showed only biochemical benefit.
In conclusion, the detoxification pathways, inflammatory pathways and some environmental sources contribute towards increased free radical load. If antioxidant defenses of the body are poor, either due to lower intake of dietary micronutrients or genetically determined low levels of endogenous antioxidants, oxidative stress may occur. Inflammatory reactions themselves induce oxidative stress by producing more and more free radicals during the respiratory burst. Oxidative stress and inflammation may comprise another chicken and egg story, each following the other. In such circumstances, supplementation with antioxidants may break the vicious cycle and help curb the progression of disease. Large clinical trials of antioxidant supplementation are necessary to explore the extent of treatment benefit and hence strengthen our hypothesis regarding antioxidant supplementation in various diseases.
References
1. Sampson HA. Food hypersensitivity: manifestations, diagnosis, and natural history. Food Tech. 1992;141–4.
2. Liska DJ. The detoxification enzyme systems. Altern Med Rev. 1998;3:187–98.
3. Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403.
4. Fiorelli G, De Feo TM, Duca L, Tavazzi D, Nava I, Fargion S, et al. Red blood cell antioxidant and iron status in alcoholic and nonalcoholic cirrhosis. Eur J Clin Invest. 2002;32 Suppl 1:21–7
5. Pemberton PW, Smith A, Warnes TW. Non-invasive monitoring of oxidant stress in alcoholic liver disease. Scand J Gastroenterol. 2005;40:1102–8
6. Masalkar PD, Abhang SA. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin Chim Acta. 2005;355:61–5
7. Seki S, Kitada T, Sakaguchi H, Nakatani K, Wakasa K. Pathological significance of oxidative cellular damage in human alcoholic liver disease. Histopathology. 2003;42:365–71
8. Stewart SF, Vidali M, Day CP, Albano E, Jones DE. Oxidative stress as a trigger for cellular immune responses in patients with alcoholic liver disease. Hepatology. 2004;39:197–203
9. Szuster-Ciesielska A, Daniluk J, Kandefer-Szerszeñ M. Oxidative stress in the blood of patients with alcohol-related liver cirrhosis. Med Sci Monit. 2002;8:CR419–24
10. Nalini G, Hariprasad C, Narayanan VA. Oxidative stress in alcoholic liver disease. Indian J Med Res. 1999;110:200–3
11. Pemberton PW, Smith A, Warnes TW. Non-invasive monitoring of oxidant stress in alcoholic liver disease. Scand J Gastroenterol. 2005;40:1102–8
12. Bhardwaj P, Madan K, Thareja S, Joshi YK, Saraya A. Comparative redox status in alcoholic liver disease and non-alcoholic fatty liver disease. Hepatology International 2008 (article in Press)
13. Chawla RK, Watson WH, Eastin CE, Lee EY, Schmidt J, McClain CJ. S-adenosylmethionine def iciency and TNF-alpha in lipopolysaccharide-induced hepatic injury. Am J Physiol. 1998;275:G125–9
14. Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A,. S-Adenosylmethionine in the treatment of alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind multicentre clinical trial. J Hepatol. 1999;30:1081–9
15. Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, et al. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand J Gastroenterol. 1989;24:407–15.
16. Garcia-Ruiz C, Morales A, Colell A, Ballesta A, Rodés J, Kaplowitz N, et al. Feeding S-adenosyl-L-methionine attenuates both ethanolinduced depletion of mitochondrial glutathione and mitochondrial dysfunct ion in per iportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–14
17. Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatit is. J Hepatol. 2007;47:277–83
18. Hill DB, Devalaraja R, Joshi-Barve S, Barve S, McClain CJ. Antioxidants attenuate nuclear factor-kappa B activation and tumor necrosis factor-alpha production in alcoholic hepatitis patient monocytes and rat Kupffer cells, in vitro. Clin Biochem. 1999;32:563–70
19. de la Maza MP, Petermann M, Bunout D, Hirsch S. Effects of longterm vitamin E supplementation in alcoholic cirrhotics. J Am Coll Nutr. 1995;14:192–6
20. Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40–6
21. Day CP, James OF. Steatohepatit is: a tale of two hits? Gastroenterology. 1998;114:842–5
22. Diehl AM. Nonalcoholic steatohepat itis. Semin Liver Dis. 1999;19:221–9
23. Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57–69
24. Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, et al. Immune response towards lipid peroxidation products as a perdictor of progression of non alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–93
25. Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol. 2006;40:930–5
26. Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S.. Vitamin E and vitamin C treatment improves fibrosis in patients with non alcoholic steatohepaitis. Am J Gastroenterol. 2003;98:2485–90
27. Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, et al. Non alcoholic fatty liver disease in an area of southern Italy: main clinical , histological and pathophysiological aspects. J Hepatology. 2001;35:568–74
28. Kawanaka M, Mahmood S, Niiyama G, Izumi A, Kamei A, Ikeda H, et al. Control of oxidative stress and reduction in biochemical markers by Vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot study. Hepatol Res. 2004;29:39–41
29. Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–8
30. Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–9
31. Boya P, Pena A, Beloqui O, Larrea E, Conchillo M, Castelruiz Y, et al. Antioxidant status and glutathione metabolism in peripheral blood mononuclear cells from patients with chronic hepatitis C. Hepatol. 1999;31:808–14
32. Pace GW, Leaf CD. The role of oxidative stress in HIV disease. Free Radic Biol Med. 1995;19:523–8
33. Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991;266:19490–8
34. Cardin R, D’Errico A, Fiorentino M, Cecchetto A, Naccarato R, Farinati F. Hepatocyte proliferation and apoptosis in relation to oxidative damage in alcohol-related liver disease.. Alcohol Alcohol. 2002;37:43–8
35. Yadav D, Hertan HI, Schweitzer P, Norkus EP, Pitchumoni CS. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol.2002;97:2634–9
36. Kawamura K, Kobayashi Y, Kageyama F, Kawasaki T, Nagasawa M, Toyokuni S, et al. Enhanced hepatic lipid peroxidation in patients with primary biliary cirrhosis. Am J Gast roenterol. 2000;95:3596–601
37. Mahmood S, Kawanaka M, Kamei A, Izumi A, Nakata K, Niiyama G, et al. Immunohistochemical evaluation of oxidative stress markers in chronic hepatitis C. Antioxid Redox Signal. 2004;6:19–24
38. Larrea E, Beloqui O, Muñoz-Navas MA, Civeira MP, Prieto J. Superoxide dismutase in patients with chronic hepatitis C virus infection. Free Radic Biol Med. 1998;24:1235–41
39. Marotta F, Yoshida C, Barreto R, Naito Y, Packer L. Oxidativeinflammatory damage in cirrhosis: ef fect of vitamin E and a fermented papaya preparation. J Gastroenterol Hepatol. 2007;22:697–703
40. Groenbaek K, Friis H, Hansen M, Ring-Larsen H, Krarup HB. The effect of antioxidant supplementation on hepatitis C viral load, transaminases and oxidative status: a randomized trial among chronic hepatitis C virus-infected patients. Eur J Gastroenterol Hepatol. 2006;18:985–9
41. Andreone P, Fiorino S, Cursaro C, Gramenzi A, Margotti M, Di Giammarino L, et al. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antiviral Res. 2001;49:75–81
42. von Herbay A, Stahl W, Niederau C, Sies H. Vitamin E improves the aminotransferase status of patients suf fering f rom viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res. 1997;27:599–605
43. Saeian K, Bajaj JS, Franco J, Knox JF, Daniel J, Peine C, et al. High-dose vitamin E supplementation does not diminish ribavirinassociated haemolysis in hepatitis C treatment with combination standard alpha-interferon and ribavirin. Aliment Pharmacol Ther. 2004;20:1189–93
44. Idéo G, Bellobuono A, Tempini S, Mondazzi L, Airoldi A, Benetti G, et al. Antioxidant drugs combined with alpha-interferon in chronic hepatitis C not responsive to alpha-interferon alone: a randomized, multicentre study. Eur J Gast roenterol Hepatol. 1999;11:1203–7
45. Look MP, Gerard A, Rao GS, Sudhop T, Fischer HP, Sauerbruch T, et al. Interferon/antioxidant combination therapy for chronic hepatitis C—a controlled pilot trial. Antiviral Res. 1999;43:113–22
46. Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66–73
47. Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6:673–84
48. Uden S, Bilton D, Guyan PM, Kay PM, Braganza JM. Rationale for antioxidant therapy in pancreatitis and cystic fibrosis. Adv Exp Med Biol. 1990;264:555–72
49. Dziurkowska-Marek A, Marek TA, Nowak A, Kacperek-Hartleb T, Sierka E, Nowakowska-Du³awa E. The dynamics of the oxidantantioxidant balance in the early phase of human acute biliary pancreatitis. Pancreatology. 2004;4:215–22
50. Tsai K, Wang SS, Chen TS, Kong CW, Chang FY, Lee SD, et al. Oxidative stress: an important phenomenon with pathogenetic signif icance in the progression of acute pancreatitis. Gut. 1998;42:850–5
51. Schoenberg MH, Birk D, Beger HG. Oxidative stress in acute and chronic pancreatit is. Am J Clin Nutr. 1995;62(6 Suppl): 1306S–1314S
52. Sajewicz W, Milnerowicz S, Nabzdyk S. Blood plasma antioxidant defense in pat ients with pancreatitis. Pancreas. 2006;32:139–44
53. Curran FJ, Sattar N, Talwar D, Baxter JN, Imrie CW. Relationship of carotenoid and vitamins A and E with the acute inflammatory response in acute pancreatitis. Br J Surg. 2000;87:301–5
54. Virlos IT, Mason J, Schof ield D, McCloy RF, Eddleston JM, Siriwardena AK. Intravenous n-acetylcysteine, ascorbic acid and selenium-based anti-oxidant therapy in severe acute pancreatitis. Scand J Gastroenterol. 2003;38:1262–7
55. Lavy A, Karban A, Suissa A, Yassin K, Hermesh I, Ben-Amotz A. Natural beta-carotene for the prevention of post-ERCP pancreatitis. Pancreas. 2004;29:e45–50
56. Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, et al. Randomized, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis Gut. 2007;56:1439–44
57. Sateesh J, Bhardwaj P, Singh N, Saraya A. Effect of antioxidant treatment on hospital stay and complications in patients with early acute pancreatitis. Ind J Gastroenterol 207;26(Suppl 2): A 104
58. Tandon RK, Sato N, Garg PK. Chronic pancreatitis: Asia-Pacific consensus report. J Gastroenterol Hepatol. 2002;17:508–18
59. Pitchumoni CS. Chronic pancreat itis: pathogenesis and management of pain. J Clin Gastroenterol. 1998;27:101–7
60. Schoenberg MH, Buchler M, Pietrzyk C, Uhl W, Birk D, Eisele S, et al. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995;10:36–43
61. Van Gossum A, Closset P, Noel E, Cremer M, Neve J. Deficiency in antioxidant factors in patients with alcohol-related chronic pancreatitis.Dig Dis Sci. 1996;41:1225–31
62. Hausmann DH, Prstmann T, Weber I, Hansmann S, Dummler W, Liebe S, et al. Cu/Zn SOD in human pancreatic tissue and juice. Intl J Pancreatol 1997; 22(3):207–213 author
63. Braganza JM. The pathogenesis of chronic pancreatitis. QJM. 1996;89:243–50
64. Bhardwaj P, Thareja S, Prakash S, Saraya A. Micronutrient antioxidant intake in patients with chronic pancreatitis. Trop Gastroenterol. 2004;25:69–72
65. Bhardwaj P, Garg PK, Saraya A. Free radical mediated oxidative stress in patients with chronic pancreatitis. Free Rad Res 2006;40(Suppl 1):S107 author
66. Uden S, Schofield D, Miller PF, Day JP Bottiglier T, Braganza JM. Aliment Pharmacol Ther. 1992;6:229–40.
67. Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, et al. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10:499–503
68. Nandi B, Garg PK, Bhardwaj P, Prakash S, Tandon RK. Efficacy of antioxidants for pain relief in patients with chronic pancreatitis: A randomized controlled trial. Ind J Gastroenterol 2002;21(suppl 1):A43 author
69. Brandtzaeg P, Haraldsen G, Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin Immunopathol. 1997;18:555–89
70. Simmonds NJ, Allen RE, Stevens TR, Van Someren RN, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inf lammatory bowel disease. Gastroenterology. 1992;103:186–96
71. Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015–21
72. Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inf lammatory bowel disease: semiquantif icat ion, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36
73. D’Odorico A, Bortolan S, Cardin R, D’Inca’ R, Martines D, Ferronato A, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–94
74. Wendland BE, Aghdassi E, Tam C, Carrrier J, Steinhart AH, Wolman SL, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr. 2001;74:259–64
75. Fernandez-Banares F, Abad-Lacruz A, Xiol X, Xiol X, Gine JJ, Dolz C, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84(7):744–8
76. Tsunada S, Iwakiri R, Ootani H, Aw TY, Fujimoto K. Redox imbalance in the colonic mucosa of ulcerative colitis. Scand J Gastroenterol. 2003;38:1002–3
77. Aghdassi E, Wendland BE, Steinhart AH, Wolman SL, Jeejeebhoy K, Allard JP. Antioxidant vitamin supplementation in Crohn’s disease decreases oxidative stress. a randomized controlled trial. Am J Gastroenterol. 2003;98:348–53
78. Barbosa DS, Cecchini R, El Kadri MZ, Rodríguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition. 2003;19:837–42
79. Geerling BJ, Badart-Smook A, van Deursen C, et al. Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohn’s disease in remission: effects on antioxidant status and fatty acid profile. Inflamm Bowel Dis. 2000;6:77–84