48uep6bbphidvals|44
48uep6bbphidcol4|ID
48uep6bbph|2000F98CTab_Articles|Fulltext
Although microscopic colitis (MC) was first described in 1976, it has absorbed the clinician and pathologist only in the last decade.(1,2,3,4,5,6) A diagnosis of MC is suspected in a patient with chronic large bowel diarrhoea, normal looking colonic mucosa on colonoscopy and in whom drug use and colonic infections have been excluded.(2,3,4,5) As the nomenclature suggests, the hallmark of a diagnosis of MC is based on microscopic abnormalities in the colonic mucosa. The histological features may include abnormalities in the colonic epithelial layer, basement membrane, number of intra-epithelial lymphocytes (IEL), types of inflammatory infiltrates in the lamina propria and the crypt architecture.(6,7) Collagenous colitis and lymphocytic colitis are the two commonly recognised types of MC . Patients not fitting into these two subtypes have generally been classified as non-specific colitis.(8) Based on the presence of a single or a combination of abnormalities, the spectrum of histological abnormalities has been extended recently and MC is now sub-classified into 5 subtypes: collagenous colitis (CC), lymphocytic colitis (LC), minimal change colitis (MCC), microscopic colitis with giant cells and microscopic colitis not otherwise specified (MC–NOS).(9,10) There is a lack of literature on MC from Asia and most studies describe only two subtypes of MC.(11,12) We, therefore,describe the spectrum of histological abnormalities in a cohort of microscopic colitis patients registered at our clinic.
MATERIAL AND METHODS
In a retrospective study, we reviewed the clinical records of 29 patients registered in our clinic with a diagnosis of MC between January 2000 and March 2006. All patients with chronic large bowel, non-bloody and painless diarrhoea (> 6months) were included. A detailed evaluation of all patients was done and parasitic and common bacterial infections were excluded by stool examination. A detailed drug history was taken. These patients underwent colonoscopic/sigmoidoscopic examination and multiple colonic mucosal biopsies were taken.
HISTOPATHOLOGY
For a detailed histological examination, 5 µm thick paraffin embedded sections of colonic biopsies were cut and stained serially with hematoxylin and eosin stains. Sirius Red and Masson trichrome staining of the sections were done to look for sub-epithelial collagen bands. Computer assisted morphometry was used in selected patients to measure the thickness of the collagen band. An image analysis system equipped with Leica (Q win) and Leica Quantimet 500 MC software (Leica, Germany) was used for this. Basement membrane measurement was performed at multiple points where three adjacent crypts were cut vertically extending all the way down to the muscularis mucosa from well-oriented sections of the biopsies. The number of intra-epithelial lymphocytes (IEL) per 100 epithelial cells was calculated by counting the number per 300 epithelial cells divided by three. Only the surface epithelium was examined and areas overlying lymph follicles in the lamina propria were avoided. Counting of IEL was performed in sections stained with haematoxylin and eosin. Grading of chronic inflammatory cells in the lamina propria was taken as mild, moderate and severe infiltration. Two pathologists independently reported all biopsy specimens and a consensus was reached for each specimen.
Diagnostic criteria for collagenous colitis(10,13) ·
Essential criteria: A diffusely distributed and thickened subepithelial collagen layer more than or equal to10 µm (in the normal colon, the width of the sub-epithelial basement membrane is generally less than 4 to 5 ìm). (Fig 1)
· An increase in the number of IEL may be present but less than 20/100 surface colonocytes
· Epithelial damage such as flattening and detachment.
· Inflammation in the lamina propria with mainly mononuclear cells.
Diagnostic criteria for lymphocytic colitis
· Essential criteria: An increase in IEL more than 20 per 100 colonocytes (Fig 2)
· A sub-epithelial collagen layer <10 µm.
· Epithelial damage
· Inflammation in the lamina propria with mainly mononuclear cells.
Diagnostic criteria for minimal change colitis (Fig 3)
· Essential criteria: Abnormalities of crypt architecture such as cryptitis, crypt abscesses, crypt dystrophy
· A sub-epithelial collagen layer <10 µm.
· An increase in the number of IEL may be present but less than 20/100 surface colonocytes
Diagnostic criteria for MC–NOS (Fig 4)
· Essential criteria: An increase in chronic inflammatory cells consisting of lymphocytes, eosinophils, plasma cells. The lamina propria was considered to have an increase of chronic inflammatory cells if the normal gradient of decrease in inflammatory cells down the lamina propria was lost and if basal plasma cells were seen at the level of the upper border of the muscularis mucosae at more than one site.
· A sub-epithelial collagen layer <10 µm.
· IEL less than 20/100 colonocytes
RESULTS
Demographic characteristics
The mean age (±SD) of the patients with MC was 38.5+15.7 years (male 75.8%). The mean age (±SD) of patients with CC, LC, MCC and MC–NOS were 40.7+13.4, 21.75+6.6, 47.1+14.1 and 37.9+16.8 years, respectively. All of them had chronic large bowel type of diarrhoea. The mean duration of disease was 88.5, 60, 52.5 and 25.8 months, respectively in patients with CC, LC, MCC and MC–NOS. Abdominal discomfort was present in 28.6%, 25%, 14.3%, and 33.3% of patients with CC, LC, MCC and MC–NOS. Weight loss was observed in 14.3%, 25%, 14.3% and 16.6% of patients with CC, LC, MCC and MC– NOS. None of them had blood stools, prolonged fever, and intestinal obstruction. The colonoscopic examination was normal in all of them.
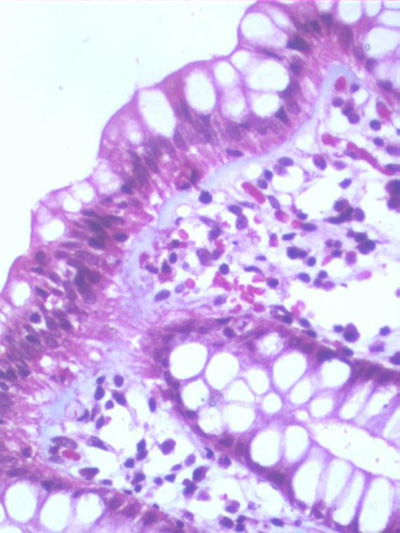
Fig 1: A photomicrograph of the colonic biopsies showing a thick collagen band in the
sub-epithelial region (Masson trichrome x20)
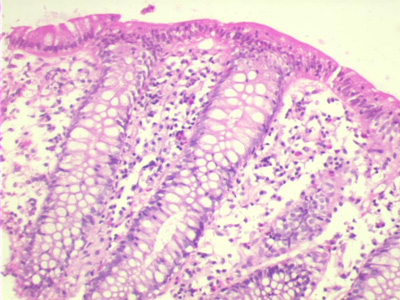
Fig 2: A photomicrograph of the colonic biopsies showing increase in intraepithelial
lymphocytes without significant sub-epithelial collagen band. There is edema and
eosinophilic infiltration in the lamina propria (hematoxylin and eosin x10)
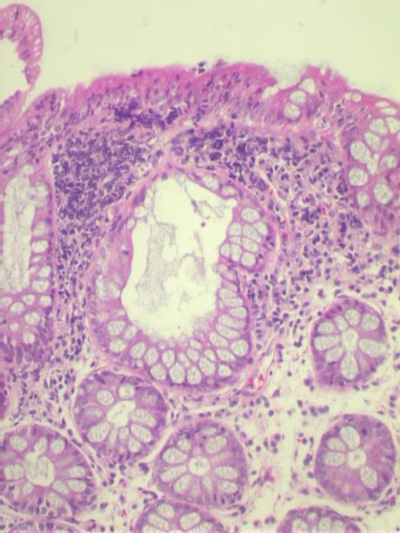
Fig 3: A photomicrograph of the colonic biopsies showing alteration in the architecture
of the crypts in the form of crypt dilatation. In addition the biopsy shows edema in the
lamina propria and a lymphoid follicle. (hematoxylin and eosin x10)
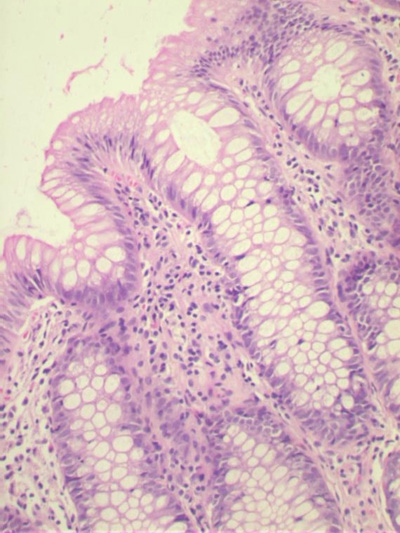
Fig 4: A photomicrograph of the colonic biopsies showing mild increase in chronic
inflammatory cells infiltrate in the lamina propria(hematoxylin and eosin x4)
Table I: Histological features in subtypes of microscopic colitis
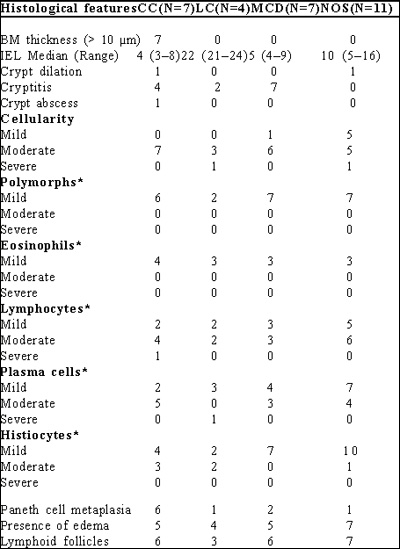
* Per 100 colonocytes
Spectrum of MC: Table 1
Collagenous colitis: Of 29 patients, 7 (24.1%) patients had CC. On morphometry, the thickness of the basement membrane varied from 10.01–11.96 µm in them. The median IEL count in them was 4 (range 3–8) per 100 colonocytes. 4 had crypt architecture abnormalities; crypt dilatation in 1, cryptitis in 4 and crypt abscess in 1. All 7 had moderate increase in cellular infiltrates in the lamina propria. 6 and 5 of them showed paneth cell metaplasia and lymphoid aggregates in the lamina propria, respectively.
Lymphocytic colitis: 4 of 29 patients with MC had LC. The median IEL/100 colonocytes was 22 (range 21–24). The thickness of the basement membrane varied from between 2.6 and 2.9 µm . None of these patients had any abnormality in crypt architecture. 3 and 1 had moderate and severe increase, respectively, in the inflammatory cell infiltrate in the lamina propria.
Minimal change colitis: 7 patients had MCC. The major histological finding in them was the presence of crypt abnormality in the form of cryptitis and crypt abscesses without any evidence of collagen band thickening. Median IEL count in them was 5 (Range 4–9). 6 and 1 had moderate and mild increase, respectively, in the cellularity of the lamina propria. 6 showed lymphoid aggregates in the lamina propria. 5 patients showed paneth cell metaplasia.
MC–NOS: 11 patients had MC–NOS. The major histological abnormality in them was an increase in the chronic mixed inflammatory infiltrate comprising lymphocytes, eosinophils, plasma cells and histiocytes. Thickness of the basement membrane was 2.64 µm . The median IEL count was 4 (range 3– 8) per 100 colonocytes. Only one showed crypt dilation.
Microscopic colitis with giant cells: No patient was observed with MC with giant cells.
Relationship between symptoms and type of Microscopic colitis:
There was no correlation between the clinical manifestations and subtypes of MC.
DISCUSSION
The histological spectrum of MC has recently widened.(9,10) In the present study including 29 patients with MC; 7 had CC, 4 had LC, 7 had MCC and 11 had MC–NOS. Recently, MC has caught the interest of clinical and basic scientists; this is reflected by an increase in the number of publications on MC during the last decade.
A significant increase in the incidence of MC during the last two decades has been reported in two large regional studies from Sweden and Minnesota.(13,14) The Swedish study including 1028 patients with non-bloody diarrhoea who underwent colonoscopic examination during 1993 through 1998, 97 (9·5%) fulfilled the diagnostic criteria of MC (51 CC and 46 LC). The incidence increased from 3.7 per 100,000 during 1993–95 to 6.1 per 100 000 during 1996–98 for CC, and from 3.1 per 100,000 to 5.7 per 100,000 for LC.14 The incidence of MC has increased slowly but continuously from 0.8 in 1985–89 to 2.6 in 1990–93, 10.3 in 1994–97, and 19.1 in 1998–2001 (all per 100000) in the US.(13)
Because the course of the disease is usually mild it is very likely that a significant proportion of patients with MC remain undiagnosed. The present incident and prevalent cases with MC may represent only the tip of the iceberg.(15,16,17,18) From the clinical point of view, in 10–20% of patients with MC, an initial diagnosis of irritable bowel syndrome is made and 15–20% of the general population has symptoms of irritable bowel syndrome. The rise in incidence of MC is apparently due to an increase in the awareness and recognition of this entity, many cases may have previously been unrecognised or erroneously diagnosed as irritable bowel syndrome.(4,15,16,17,18)
Both CC and LC are diseases of the elderly, with reported mean age of diagnosis varying between the early fifties and the mid sixties for both disorders.(2,3,4,5) Both conditions have also been described in children. Most patients in the present study were relatively younger, the reason for which is not clear. In another study from the Indian subcontinent, Satarasinghe, et al observed the mean age in their 29 patients with CC as 45+7 years (range 21–50).(12)
The pathological changes seen in the colonic mucosa in patients with MC may be because of two reasons. Firstly the changes, such as increase in chronic inflammatory cells in the lamina propria, may represent the response of the colonic mucosa to unknown inciting stimuli. Secondly, some of the colonic mucosal changes seen in patients with MC may well be a specific response to the inciting agent. As histological changes in MC vary from an increase in IEL in LC, thickening of the basement membrane in CC and damage to colonic crypts in MCC, the factors which direct these changes is not known.(5,6,7) Similarity in clinical presentation, natural history and response to treatment, makes us wonder if they are the different presentations of the same disease where the colonic tissue reacts differently to the same aetiological stimuli.
Terms such as “unspecific chronic inflammation” or “nonspecific colitis” should be avoided so as to include patients in proper therapeutic groups. By considering the clinical history and symptoms, the pathologist should be able to reach the correct diagnosis in most cases.(8) Good communication between the pathologist and clinician is therefore essential.
The symptoms of MC are often disabling, and the current treatment is far from optimum.(2,3,4,5) First, awareness of clinical and pathological spectra of this condition is essential. Second, it is important to obtain colonic biopsies for histological examination even when colonic mucosa appears normal at colonoscopic examination if clinical history is suggestive. Third, because both the density of intraepithelial lymphocytes and the thickness of the collagenous band tend to be patchy, it is essential to take multiple biopsies throughout the colon.(19,20) Involvement of the left colon seems to be less intense than that of the right, and biopsies taken only from the left part may result in missed diagnosis in about 10% of cases.(2,3,4,5,6) It is therefore recommended that tissue samples be obtained from the transverse or ascending colon to definitely rule out collagenous colitis. The natural history of microscopic colitis is benign and the course of the disease is variable with alternating spontaneous remissions and relapse.(2) LC is more likely to undergo clinical remission than CC. A few patients with MC have been reported to develop Crohn’s disease or ulcerative colitis, but it is debatable whether this is true evolvement, initial misdiagnosis, or variation in the histopathology of microscopic colitis.21,22,23 There is high spontaneous resolution of symptoms, and only a few controlled trials in CC have been done. Therefore, the treatment of microscopic colitis is largely empirical.(2,3,4,5 24,25,26) A relevant therapeutic approach includes elimination of intake of secretagogues such as caffeine and non-steroidal anti-inflammatory drugs. However, a recent Cochrane review suggests that budesonide (three 3 mg tablets daily) and bismuth subsalicylate (nine 262 mg tablets daily in three divided doses) is effective in the initial treatment of CC.(24,25)
In conclusion, the spectrum of microscopic colitis is wide. Both the gastroenterologist and the pathologist must be aware of these diagnoses while evaluating patients with persistent non-bloody watery diarrhoea.
REFERENCES
1. Lindström CG. Collagenous colitis with watery diarrhea-a new entity? Pathol Eur. 1976;11:87–9.
2. Loftus EV. Microscopic colitis: epidemiology and treatment. Am J Gastroenterol. 2003;98:S31–6.
3. Pardi DS. Microscopic colitis: an update. Inflamm Bowel Dis. 2004;10:860–70.
4. Nielsen OH, Vainer B, Schaffalitzky de Muckadell OB. Microscopic colitis: a missed diagnosis? Lancet. 2004;364:2055–7.
5. Nyhlin N, Bohr J, Eriksson S, Tysk C. Systematic review: microscopic colitis. Aliment Pharmacol Ther. 2006;23:1525–534.
6. Tagkalidis P, Bhathal P, Gibson P. Microscopic colitis. J Gastroenterol Hepatol. 2002;17:236–48.
7. Liszka L, Woszczyk D, Pajak J. Histopathological diagnosis of microscopic colitis. J Gastroenterol Hepatol. 2006;21:792–7.
8. Geboes K, Villanacci V. Terminology for the diagnosis of colitis. J Clin Pathol. 2005;58:1133–4.
9. Libbrecht L, Croes R, Ectors N, Staels F, Geboes K. Microscopic colitis with giant cells. Histopathology. 2002;40:335–8.
10. Warren BF, Edwards CM, Travis SPL. Microscopic colitis: classification and terminology. Histopathology. 2002;40:374–6.
11. Garg PK, Singh J, Dhali GK, Mathur M, Sharma MP. Microscopi colitis is a cause of large bowel diarrhea in Northern India. J Clin Gastroenterol. 1996;22:11–5.
12. Satarasinghe RL, Fernando HR, Jayamaha DH, Samarasinghe I, De Silva AP. Collagenous colitis in adult Sri Lankans: experience from the Indian subcontinent. Gut. 2006;55:436.
13. Pardi DS, Loftus EV Jr, Smyrk TC, Kammer PP, Tremaine WJ, Schleck CD, et al. The epidemiology of microscopic colitis: a population-based study in Olmsted County, Minnesota. Gut. 2006;56:504–8.
14. Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Microscopic colitis: a common diarrhoeal disease: An epidemiological study in Örebro, Sweden, 1993–1998. Gut. 2004;53:346–50.
15. Kitchen PA, Levi AJ, Domizio P, Talbot IC, Forbes A, Price AB. London Inflammatory Bowel Disease Forum. Microscopic colitis: the tip of the iceberg? Eur J Gastroenterol Hepatol. 2002;14:1199– 204.
16. Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006;48:116–32.
17. Limsui D, Pardi DS, Camilleri M, Loftus Jr EV, Kammer PP, Tremaine WJ et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2006;13;175–81.
18. Schiller LR. Diagnosis and management of microscopic colitis syndrome. J Clin Gastroenterol. 2004;38:S27–30.
19. Tremaine WJ. Diagnosing collagenous colitis: does it make a difference? Eur J Gastroenterol Hepatol. 1999;11:477–9.
20. Jaskiewicz K, Rzepko R, Adrych K, Smoczynski M. Microscopic colitis in routine colonoscopies. Dig Dis Sci. 2006;51:241–4.
21. Germain E, Roblin X, Colle PE, Barnoud R, Faucheron JL, Bonaz B. Primary ileal villous atrophy associated with microscopic colitis. J Gastroenterol Hepatol. 2005; 20:1800–1.
22. Pokorny CS, Kneale KL, Henderson CJ. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol. 2001;32:435–8.
23. O’Beirne JP, Ireland A. Progression of collagenous colitis to Crohn’s disease. Eur J Gastroenterol Hepatol. 2005;17:573–5.
24. Fine KD, Lee EL. Efficacy of open-label bismuth subsalicylate for the treatment of microscopic colitis. Gastroenterology. 1998;114:29–36.
25. Chande N, McDonald JW, Macdonald JK. Interventions for treating lymphocytic colitis: Cochrane Database Syst Rev. 2007;CD006096. 26. Wildt S, Munck LK, Vinter-Jensen L, Hanse BF, Nordgaard-Lassen I, Christensen S, et al. Probiotic treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis. Inflamm Bowel Dis. 2006;12:395–401.