Ankur Gupta, Uday C Ghoshal, Samir Mohindra, Vivek A Saraswat
Department of Gastroenterology,
Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Raebareli Road, Lucknow-226104, India
Corresponding Author:
Dr. Uday C Ghoshal
Email: udayghoshal@gmail.com
48uep6bbphidvals|658 48uep6bbph|2000F98CTab_Articles|Fulltext Acute pancreatitis, a common gastrointestinal emergency, is often caused by gallstones and alcohol.[1] Medications are infrequent but important causes for acute pancreatitis. Olanzapine, a dopaminergic and serotoninergic receptor antagonist, is an atypical anti-psychotic agent of thienobenzodiazepine class and is known to have several side effects. It has been rarely reported to cause acute pancreatitis.[2-8] We report a patient with acute necrotizing pancreatitis probably induced by olanzapine and review reports of this rare condition from the literature.
Case report
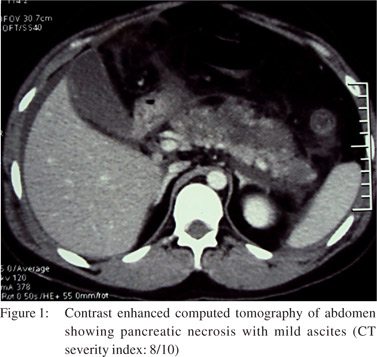
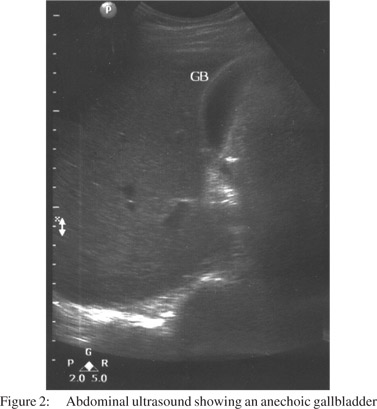
A 25-year-old man presented with sudden onset, severe epigastric pain radiating to his back, accompanied by bilious vomiting. Investigations, after 12 hours of onset of pain, revealed a serum amylase level of 2300 units per litre (normal: 23 to 85 U/l), total leucocyte count (TLC) 14700/mm3 (normal: 4,000-10,000 leucocytes/mm3) with 94% neutrophils. He had paralytic ileus, which improved over next three days. There was no oliguria, gastrointestinal bleed or altered sensorium. Contrast enhanced computed tomography (CECT) of the abdomen (Figure 1) on day 3 of illness, showed necrotizing pancreatitis with mild ascites with a CT severity index of 8/10.[9] The patient was being treated with olanzapine (10 mg/d) for ‘psychosis, not otherwise specified’, since seven months. He was a non-smoker, non-alcoholic, and did not take any other medication or suffered any other illness. The patient presented to our centre on 11th day of illness with respiratory distress and fever. His PO2 while on room air was 68 mmHg and TLC was 22800/mm3 (80% neutrophils). Other investigations included serum creatinine: 0.9 mg/dl (normal: 0.8 to 1.4 mg/dl), bilirubin: 1.1 mg/dl (normal: 0.3 to1.5 mg/dl), triglycerides: 162 mg/dl (normal: less than 150 mg/dl) and serum calcium: 8.3 mg/dl (normal: 8 to 10 mg/dl). An abdominal ultrasound showed normal gall bladder (Figure 2) and bile duct. He was successfully treated by discontinuing olanzapine therapy, and was instituted on parenteral imipenem, cilastatin and oxygen inhalation and was later discharged from the hospital. He remained well during a follow-up period of 2 months.
Discussion
Our patient had acute pancreatitis as evidenced by typical pain, elevated serum amylase and supportive imaging.[10] It was most likely caused by olanzapine given its temporal association with the illness, absence of other etiological and risk factors, improvement on discontinuation of the drug and its known association with acute pancreatitis, even though rare. Though, recurrence on re-challenge would have confirmed the association, it was not ethical.
Using Naranjo causality scale[11] acute pancreatitis in our patient qualified as a probable adverse drug reaction to olanzapine. Koller et al[12] studied anti-psychotic associated pancreatitis and noted that 32 of 51 (63%) patients with pancreatitis thought to be due to olanzapine had 6 months or less of drug exposure before developing pancreatitis, which is somewhat similar to the duration of exposure in our patient. Olanzapine is a commonly used drug in psychiatry. In an Indian survey, 30% of prescriptions for psychosis contained olanzapine.[13] In another outpatient survey, olanzapine accounted for 609 of 1477 (41.2%) prescriptions for psychosis.[14]
Similar prescription patterns have been reported from the West.[15] Considering the above data on frequency of olanzapine use and rarity of reports of pancreatitis associated with this drug, it seems like an uncommon complication; however, underreporting may also be a confounder.
We found seven reports on ten patients of acute pancreatitis associated with olanzapine use, in the English medical literature. Male to female ratio is 6:4. Six of these 10 patients had concomitant risk factors such as other medications, alcohol consumption or pre-existing hypertriglyceridemia, hence it is difficult to incriminate olanzapine as the only agent inciting pancreatitis in these patients. Duration of olanzapine use varied from 6 days to 60 months. Three of these patients had hypertriglyceridemia. In one of the patients, hypertriglyceridemia was shown to improve on follow-up, after olanzapine was stopped. This might suggest that hypertriglyceridemia may be one of the mechanisms for olanzapine induced acute pancreatitis.
We conclude that olanzapine is a rare cause of acute pancreatitis. Olanzapine induced acute pancreatitis is reported more often in males and can occurs 6 days to 60 months after continued intake of the drug. Most of the patients recover with cessation of olanzapine and supportive management.
Although the definite mechanism of this drug-induced illness is not known, some patients have been reported to have hypertriglyceridemia. Awareness about this entity is important while treating patients with olanzapine.
References
- Thomson SR, Hendry WS, McFarlane GA, Davidson AI. Epidemiology and outcome of acute pancreatitis. Br J Surg. 1987;74:398–401.
- Doucette DE, Grenier JP, Robertson PS. Olanzapine-induced acute pancreatitis. Ann Pharmacother. 2000;34:1128–31.
- Hagger R, Brown C, Hurley P. Olanzapine and pancreatitis. Br J Psychiatry. 2000;177:567.
- Waage C, Carlsson H, Nielsen EW. Olanzapine-induced pancreatitis: a case report. JOP. 2004;5:388–91.
- Kerr TA, Jonnalagadda S, Prakash C, Azar R. Pancreatitis following Olanzapine Therapy: A Report of Three Cases. Case Rep Gastroenterol. 2007;1:15–20.
- Kahn D, Bourgeois JA. Acute pancreatitis and diabetic ketoacidosis in a schizophrenic patient taking olanzapine. J Clin Psychopharmacol. 2007;27:397–400.
- Rossor AM, Leech N, Neely RD. Olanzapine-induced chylomicronemia presenting as acute pancreatitis. J Clin Psychopharmacol. 2007;27:395–6.
- Bracamonte JD, Underhill M, Sarmiento P. Acute pancreatitis associated with lisinopril and olanzapine. Am J Health Syst Pharm. 2010;67:214–6.
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–6.
- Bradley EL, 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–90.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
- Koller EA, Cross JT, Doraiswamy PM, Malozowski SN. Pancreatitis associated with atypical antipsychotics: from the Food and Drug Administration’s MedWatch surveillance system and published reports. Pharmacotherapy. 2003;23:1123–30.
- Grover S, Avasthi A. Anti-psychotic prescription pattern: A preliminary survey of Psychiatrists in India. Indian J Psychiatry. 2010;52:257–9.
- Grover S, Kumar V, Avasthi A, Kulhara P. An audit of first prescription of new patients attending a psychiatry walk-inclinic in north India. Indian J Pharmacol. 2012;44:319–25.
- Centorrino F, Fogarty KV, Sani G, Salvatore P, Cimbolli P, Baldessarini RJ. Antipsychotic drug use: McLean Hospital, 2002. Hum Psychopharmacol. 2005;20:355–8.
|