Sangey C Lamtha,1 VK Dixit,1 AK Jain,1 Mohan Kumar,2 Manish Kumar Tripathi,1 Pankaj Kaushik,1 Sundeep Goyal,1 Jayant Ghosh,1 Sumit Rungta1
Departments of Gastroenterology1
and Pathology,2
Institute of Medical Sciences,
Banaras Hindu University (BHU),
Varanasi, Uttar Pradesh - 221005,
India
Corresponding Author:
Dr. Sangey C Lamtha
Email: sangey79@yahoo.com
Abstract
Background: The relationship between age and serum HBV DNA levels with histological activity in chronic hepatitis B inactive carriers is still unclear. We evaluated the correlation between age and hepatitis B viral DNA levels with Metavir score in inactive chronic HBV carriers.
Methods: 50 patients (30 males and 20 females) were enrolled in the study after informed consent. Their blood samples were taken for routine investigations and specific tests for the study. Serum HBV DNA levels were quantified by real-time PCR. Metavir score was used for histologic grading.
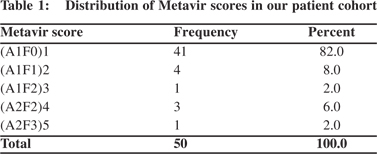
Results: A1F0, A1F1, A1F2, A2F2 and A2F3 metavir scores were found in 41 (82%), 4 (8%), 1 (2%), 3 (6%), and 1 (2%) patients, respectively. There was significant correlation between age >40 years and Metavir scores (p <0.001). However there was no significant correlation between HBV DNA level with Metavir score (p=0.074).
Conclusion: Inactive carriers of 40 years of age or more should undergo liver biopsy to look for presence of significant histological findings despite having low HBV DNA level and normal SGPT level.
|
48uep6bbphidvals|626 48uep6bbph|2000F98CTab_Articles|Fulltext Chronic hepatitis B virus (HBV) infection affects more than 400 million people globally, of which 75% are Asians.[1,2] Inactive carriers form the largest group of chronic HBV infected patients with a burden of around 300 million people. India falls in an intermediate risk group having an estimated 4-6% persons affected with hepatitis B infection. The diagnosis of the chronic HBV carrier state requires that the HBV surface antigen (HBsAg) be present in serum for 6 months. Approximately 20- 30% of persons in inactive HBsAg carrier state may undergo spontaneous reactivation of hepatitis B during follow-up.
Multiple episodes of reactivation or sustained reactivation can cause progressive hepatic damage and even hepatic decompensation, and may sometimes mimic acute viral hepatitis.
The natural history of the chronic carrier state is not completely understood. Sufficient data is not available on age, hepatitis B virus deoxyribonucleic acid (HBV-DNA) levels and histological scores from our country. The aim of this study was to examine any correlation between age, HBV DNA levels and Metavir score in inactive chronic HBV carriers.
Methods
This prospective study was conducted from April 2011 to September 2012 at our Department of Gastroenterology. The cases were enrolled from the outpatient department.
Inclusion criteria
HBsAg positive patients who were hepatitis B ‘e’ antigen (HBeAg) negative, anti-hepatitis B ‘e’ antibody (anti-HBe Ab) positive, with normal alanine aminotransferase (ALT) levels and HBV DNA level <2000 IU/mL (10000 copies/ml) were included in the study. Patients suffering from other hepatotropic viruses, metabolic liver diseases, autoimmune hepatitis, hepatocellular carcinoma (HCC), significant comorbidities and coagulopathies were excluded. Eighty incidentally detected HBsAg positive patients with normal ALT levels were initially included. However, 20 patients had HBV DNA level >2000 IU/ mL and 10 patients were hepatitis B ‘e’ antigen (HBeAg) positive. Hence they were excluded. So a total of 50 patients were finally included in the study after informed consent. Their blood samples were taken for routine and specific tests. All patients underwent liver biopsy as per standard protocol.[3] The study was approved by the Ethical Committee of Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. A detailed history, clinical examination, hemogram, liver function tests (LFT), kidney function tests (KFT), prothrombin time/ international normalized ratio (PT/INR), ultrasonography whole abdomen, and upper gastrointestinal endoscopy (UGIE) were done in each patient. All patients were tested for the following viral markers using commercially available enzyme linked immune sorbent assay (ELISA) kits: HBsAg, antihepatitis C virus (HCV) antibodies (ERBA Diagnostics, Transasia Biomedicals Limited, Daman); HBeAg, anti-HBe antibodies, IgM anti-hepatitis A virus (HAV) antibodies, IgM anti-hepatitis E virus (HEV) antibodies (Dia Pro Diagnostics, Bioprobes srl, Milano, Italy); and IgM anti-hepatitis B core antigen (HBc) antibodies (Organics Limited, Yavne, Israel).
Extraction of hepatitis B virus DNA
HBV DNA was extracted from 200 mL of serum using pure viral nucleic acid kit, as instructed by manufacturers (Roche Diagnostics, Mannkeim Germany). In brief, 200 ml serum was mixed with 200 ml of nucleic acid binding buffer (10 mmol/L Tris HCl, 10 mmol/L urea, 20% Tritone X, 200 mg poly (A) carrier ribonucleic acid (RNA), and 0.8 mg of proteinase K). The above mixture was incubated at 72°C for 10 min. 500 ml of inhibitor remover consisting of 100% ethanol, was added to the mix. The mixture was poured in columns provided and was centrifuged. The columns were washed with buffer containing 100% ethanol, 20 mmol/L NaCl and 2 mmol/L Tris HCl. Finally the DNA was eluted in 50 mL of elution buffer and was stored.
Real-time quantitative PCR for HBV DNA detection and liver biopsy
The extracted DNA was employed for quantitative HBV DNA estimation. The World Health Organization (WHO) international standards for HBV DNA quantification used for the assay were procured from the National Institute of Biological Standards and Control (NIBSC, Code 97/750, UK). Serum HBV DNA was quantified by using real-time polymerase chain reaction (RTPCR) (MiniopticonTM, Biorad, USA). The PCR assay used 10 ml of extracted template DNA and the Geno-sense kit (Genome Diagnostics, New Delhi, India) for Taqman probe assay. HBVspecific primers were derived from the conserved core/ precore region of the HBV genome. The highest and lowest detection limit of this assay was 2.5´108 and 10 IU/ml, respectively.
Liver biopsy was done after taking written consent. Under aseptic precautions and local anesthesia a liver biopsy gun (BARD) of 16 G size was used for the procedure. Liver biopsy was done under ultrasound guidance in our department. The sample was collected in 10% formalin and was sent to our Department of Pathology for histopathology using haematoxylin and eosin and reticulin staining for assessing fibrosis. Liver biopsy was assessed using the Metavir score which takes into account inflammatory activity and fibrosis.
Statistical analysis
Data were analyzed using SPSS v16.0. Differences between different groups were computed using Student’s t test and a p value of <0.05 was taken as significant.
Results
Fifty HBsAg positive patients but HBeAg negative and with HBV DNA levels <2000 IU/ml or 10,000 copies/ml were enrolled after informed consent. The patients were divided into two groups, (1) age <40 years, and (2) age e”40 years. Twenty-five patients (50%) were below 40 years of age and the remaining 25 (50%) were e”40 years. The median age of the cohort was 39 years. Most of the patients were in age groups of 22-29 and 40- 45 years. There were 30 male (60%) and 20 female patients (40%). Quantitative HBV DNA estimation revealed 41 patients with HBV DNA level <1000 IU/mL and 9 patients with >1000 IU/mL but <2000 IU/mL of HBV DNA.
Histopathology
Histopathology was done to get every patient’s Metavir score (Table 1). A Metavir score of A1F0 was present in 41 (82%) patients. These cases had mild portal inflammation and no fibrosis. A1F1 score was present in 4 patients (8%) characterized by mild portal inflammation and periportal fibrosis. There was 1 patient (2%) with A1F2 Metavir score with histological evidence of mild portal inflammation and periportal fibrosis extending into few septa. A2F2 score was present in 3 patients (6%) with features of moderate portal inflammation and few portal fibrotic septations. A2F3 was present in only 1 patient (2%) with severe portal inflammatory changes characterized by marked infiltration of monocytes, neutrophils, macrophages and marked periportal fibrosis and bridging fibrosis.
Correlation of age with Metavir score
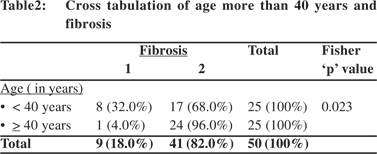
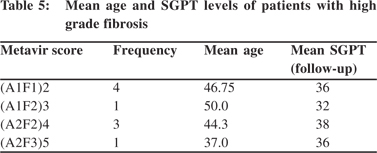
There was significant correlation between age >40 years with the Metavir score (p <0.001, and T-valve of -8.865) (Table 2). The Fisher p value was found to be 0.023 showing significant association between age >40 years and fibrosis (Table 3). The odds ratio was 11.2 implying higher odds of fibrosis in persons >40 years of age. The sensitivity, specificity, PPV and NPV were 88.8%, 58.54%, 32% and 96%, respectively. The mean age of the patients having higher grade of fibrosis is shown in Table 4.
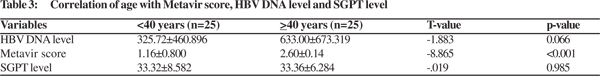

Correlation between HBV DNA level, Metavir scores and SGPT level
There was no significant correlation of age with HBV DNA level and SGPT level (Table 2). There was no significant correlation found between HBV DNA level with Metavir score and SGPT levels (p=0.074; T-value: -1.826) (Table 5).
Discussion
There is sparse data on the natural course of chronic hepatitis B virus infection in asymptomatic carriers. In this study we investigated the correlation of age and histological findings with HBV DNA levels in inactive HBV carriers. The study subjects were followed-up for 18 months. Recent developments in molecular biology have shifted HBV diagnosis from serology to molecular diagnostics. HBV DNA detection and quantification are fast becoming essential for assessing viral activity and response to therapy. Previous studies have demonstrated that HBV DNA levels are usually in excess of 105 copies in chronic hepatitis B (CHB) patients who are HBeAg positive.[4] Chronic hepatitis B patients who are HBeAg negative have a lower DNA load as compared to those who are positive. But their loads are higher than that in inactive carriers.[5] A cut-off of 20,000 IU/mL serum had been recommended to differentiate between carriers and patients with chronic disease.[6] However, a study from Greece suggested that a cut-off of e”30,000 copies/ml (6,000 IU/mL) offered higher sensitivity for correctly differentiating ‘e’ antigen negative chronic hepatitis from HBV carriers.[7] Even a cut-off of 30,000 copies/ml tends to misclassify 7% inactive carriers and 30% HBeAg-negative chronic hepatitis patients, where testing was done only once. Most of the previous investigators defined HBV carriers based on persistently normal ALT levels without evaluating their liver biopsy.[4]
Sarin et al (2008) examined 1387 incidentally detected asymptomatic HBsAg positive patients. The study highlighted that labeling of HBV infected patients as inactive carriers based on ALT and HBV DNA levels can be misleading because a significant proportion of cases with normal ALT and low HBV DNA would show advanced liver disease if a liver biopsy was performed.[8] The level of HBV viremia in different clinical forms of HBV infection are not known. Another lacuna in existing knowledge is the inconsistent correlation between HBV viremia level and histology or ALT.[9] Kam et al (1982) studied 16 inactive HBV carriers. None of them had entirely normal liver histology and four showed a slight elevation of serum transaminase activity.[10] These findings emphasize that even in asymptomatic carriers, persistent infection with HBV usually results in structural and at times functional evidence of hepatic disease.[11,12] However, the extent of these abnormalities did not correlate with the levels of detectable HBV DNA in serum or liver. Our study also found that there was no correlation between histology findings and HBV DNA levels.
In another study investigating liver biopsies of 167 chronic HBV carriers, along with a follow-up for 4 years, the authors concluded that irrespective of patient’s viral load 72.67% (117/ 161) of CHB patients with persistently normal aminotransferase levels showed significant liver histopathology changes.[13] So liver biopsy should be considered in CHB patients with persistently normal aminotransferase levels, especially those >40 years of age. Our data shows that inactive HBV carriers even with low viral loads and normal alanine aminotransferase levels can show significant histology changes especially in those aged >40 years. Inactive carriers can have normal histology in 7.5-32% cases, mild inflammation in 50%, significant liver histology in 18-25% which includes chronic persistent hepatitis in 14-19%, chronic active hepatitis in 3- 6%, cirrhosis in 1.6% and HCC in 0-0.2%. This remains unchanged in 73.2%, improves in 5.4% and worsens in 21.4% over long term follow-up.[14] In a study on 132 HBeAg negative, chronic HBV patients, who had normal alanine transferase levels, and a mean age of 36 years, the authors concluded that chronic HBV infection with persistently normal ALT is a dynamic process and not necessarily a benign disease. Serum HBV DNA and ALT levels are not enough to differentiate histologically active liver disease from inactive form.[15]
Conclusion
Inactive carriers aged more than 40 years should undergo liver biopsy to screen for significant grade inflammation and fibrosis despite having low HBV DNA and normal SGPT levels. However, large randomized studies are needed to further validate our findings and to guide the management of these inactive carriers.
References
- Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45.
- Lai CL. Chronic hepatitis B in Hong Kong: immunization strategies for the control of hepatitis B virus infection. In: Zuckerman, editor. Hepatitis B in the Asian-Pacific region vol. 1 screening, diagnosis and control. London: Royal College of Physicians; 1997.p.79–87.
- Padia SA, Baker ME, Schaeffer CJ, Remer EM, Obuchowski NA, Winans C, et al. Safety and efficacy of sonographic-guided random real-time core needle biopsy of the liver. J Clin Ultrasound. 2009;37:138–43.
- Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology. 2002;36:1408–15.
- Lok AS, McMahon BJ, Practice Guidelines Committee American Association for the Study of Liver Diseases. Chronic hepatitisB. Hepatology. 2001;34:1225–41.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology. 2001;120:1828–53.
- Manesis EK, Papatheodoridis GV, Sevastianos V, Cholongitas E, Papaioannou C, Hadziyannis SJ. Significance of hepatitis B viremia levels determined by a quantitative polymerase chain reaction assay in patients with hepatitis B e antigen-negative chronic hepatitis B virus infection. Am J Gastroenterol. 2003;98:2261–7 .
- Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virusinfected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376–84 .
- Madan K, Batra Y, Jha JK, Kumar S, Kalra N, Paul SB, et al. Clinical relevance of HBV DNA load in patients with chronic hepatitis B infection. Trop Gastroenterol. 2008;29:84–90.
- Kam W, Rall LB, Smuckler EA, Schmid R, Rutter WJ. Hepatitis B viral DNA in liver and serum of asymptomatic carriers. Proc Natl Acad Sci U S A. 1982;79:7522–6.
- Sampliner RE, Hamilton FA, Iseri OA, Tabor E, Boitnott J. The liver histology and frequency of clearance of the hepatitis B surface antigen (HBsAg) in chronic carriers. Am J Med Sci. 1979;277:17–22.
- Ganem D. Persistent infection of humans with hepatitis B virus: mechanisms and consequences. Rev Infect Dis. 1982;4:1026–47.
- Yu JG, Shang QH, An Y, Bai W, Zhang W, Yang W, et al. Study of liver biopsy in chronic HBV carriers. The 20th Conference of the Asian Pacific Association for the Study of the Liver. Hepatol Int. 2010;4:94–345.
- Bortolotti F, Jara P, Crivellaro C, Hierro L, Cadrobbi P, Frauca E, et al. Outcome of chronic hepatitis B in Caucasian children during a 20-year observation period. J Hepatol. 1998;29:184–90.
- Montazeri G, Rahban M, Mohamadnejad M, Zamani F, Hooshyar A, Fazlolahi A, et al. Liver histology and HBV DNA levels in chronically HBV infected patients with persistently normal alanine aminotransferase. Arch Iran Med. 2010;13:193–202.
|