|
Ragesh Babu Thanassery1, Saroj Kant Sinha1, Thakur Deen Yadav2, Anil D Galle1, Kim Vaiphei3, Kartar Singh1
Departments of Gastroenterology1, General Surgery2 and
Histopathology3, Postgraduate Institute of Medical Education and
Research. Chandigarh- 160012, India.
Corresponding Author:
Dr. Ragesh Babu Thandassery
Email: doc.ragesh@gmail.com
48uep6bbphidvals|615 48uep6bbph|2000F98CTab_Articles|Fulltext Endometriosis refers to the presence of endometrial stroma and glands outside the uterine cavity and musculature.[1] Ectopic implants can occur anywhere in the body. Intraperitoneal sites include the ovaries (30%), uterosacral and large ligaments (18%–24%), fallopian tubes (20%) and gastrointestinal tract. Extraperitoneal sites include the cervix (0.5%), vagina and abdominal scars.[2] Endometriosis is diagnosed in 1% of patients undergoing major surgery for any gynaecological indication.[3] Endometriosis has protean clinical manifestations that include pelvic pain (often more severe during menstruation), dysmenorrhoea, infertility, deep dyspareunia, cyclical bowel or bladder symptoms and abnormal menstrual bleeding. Symptoms are initially cyclical (40%) and become permanent when the lesions progress. Bowel endometriosis (BE) can mimic irritable bowel syndrome, infectious diseases, ischaemic enteritis/colitis, inflammatory bowel disease and neoplasms. BE presents with relapsing bouts of abdominal pain, abdominal distention, tenesmus, constipation, diarrhoea, rectal bleeding and pain during defaecation.[3] BE should be suspected in young, nulliparous patients with abdominal pain, cyclical symptoms in the initial phase along with signs of obstruction.
Case report
A 43-year-old woman presented with recurrent periumbilical colicky pain for the past 6 months, associated with bilious vomiting and constipation, each episode lasting for about 1 week. There was no past history of diarrhoea, gastrointestinal bleed, jaundice, fever, weight loss or surgery. She had two children and her menstrual cycles were regular.
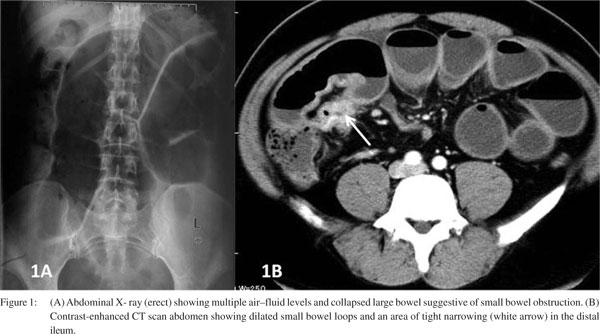
General physical examination was unremarkable. Abdominal examination showed generalized distension with visible intestinal peristalsis. There was epigastric and periumbilical tenderness. Investigations revealed normal haemogram and liver and renal function tests. Abdominal X-ray showed multiple air–fluid levels with prominent proximal small bowel loops suggestive of distal small bowel obstruction. Abdominal contrast–enhanced computed tomography (CT) scan showed similar findings with a short segment distal ileal stricture (Figure 1).
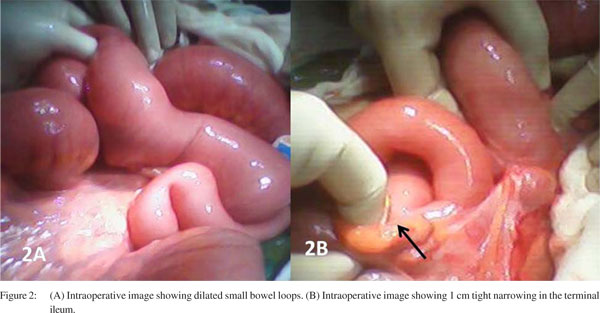
On laparotomy there were dilated proximal small bowel loops, a short segment (1 cm) tight stricture 5 cm proximal to the ileocaecal junction and a few small lymph nodes in the mesentry (Figure 2). She underwent resection of stricture end ileostomy and excision of the mesenteric lymph nodes. The histopathology of resected ileal segment showed invasion of muscularis propria by the endometrial glands and minimal chronic inflammatory cell infiltration and fibrosis suggestive of intestinal endometriosis (Figure 3). The lymph nodes showed reactive hyperplasia. Two months later, she underwent bowel restoration during which extensive search for peritoneal or mesenteric endometrial deposits was done. No similar lesions could be identified. She is asymptomatic after 2 years of followup.
 Discussion
Intestinal or bowel endometriosis (BE) is diagnosed when endometrial-like glands and stroma infiltrate the bowel wall reaching at least the subserosal fat tissue or adjacent to the neurovascular branches (subserous plexus). Endometriotic foci located on the bowel serosa should be considered peritoneal and not bowel endometriosis.[4] Gastrointestinal involvement in endometriosis is found in 3%–37% of patients.[5] Sigmoid colon, rectum and terminal ileum are the usual sites with rectosigmoid colon being the most common site (70%) and small bowel involvement, usually confined to the distal ileum as in our case is very rare (1%–7%).[6] Jubyanik and Comite described a case series of 1000 women with gynaecological complaints who underwent surgery. Endometriosis was diagnosed in 18% of them and isolated small bowel involvement was seen in only one patient.[7] Anaf et al. described BE as an “infiltration or invasion phenomenon”, and found that there is a histological continuity between superficial and underlying deep lesions of the large bowel wall suggesting lesions originate from the serosa and thereafter invade the muscularis propria. Mucosa is rarely involved as it is poorly innervated.[8] Endometriosis infiltrating the muscularis propria may lead to localized fibrosis in the bowel wall, strictures, and small or large bowel stenosis.[9]
Discussion
Intestinal or bowel endometriosis (BE) is diagnosed when endometrial-like glands and stroma infiltrate the bowel wall reaching at least the subserosal fat tissue or adjacent to the neurovascular branches (subserous plexus). Endometriotic foci located on the bowel serosa should be considered peritoneal and not bowel endometriosis.[4] Gastrointestinal involvement in endometriosis is found in 3%–37% of patients.[5] Sigmoid colon, rectum and terminal ileum are the usual sites with rectosigmoid colon being the most common site (70%) and small bowel involvement, usually confined to the distal ileum as in our case is very rare (1%–7%).[6] Jubyanik and Comite described a case series of 1000 women with gynaecological complaints who underwent surgery. Endometriosis was diagnosed in 18% of them and isolated small bowel involvement was seen in only one patient.[7] Anaf et al. described BE as an “infiltration or invasion phenomenon”, and found that there is a histological continuity between superficial and underlying deep lesions of the large bowel wall suggesting lesions originate from the serosa and thereafter invade the muscularis propria. Mucosa is rarely involved as it is poorly innervated.[8] Endometriosis infiltrating the muscularis propria may lead to localized fibrosis in the bowel wall, strictures, and small or large bowel stenosis.[9]
Malignancy has been reported in 0.7%–1% of patients with bowel endometriosis, which include endometrioid carcinoma (75% rectosigmoid), mixed mullerian tumours (80% ileocaecal) and stromal sarcoma.[10]
Acute obstruction of the bowel lumen, as in our case, occurs in <1% of patients.[11] de Bree et al. described the incidence of intestinal resection for bowel obstruction to be 0.7% among patients undergoing surgical treatment for abdominopelvic endometriosis.[12] Mussa et al. reported a case of small bowel endometriosis with intestinal obstruction, protein-losing enteropathy and anasarca.[13] Wong et al. described a case of endometriosis of the small bowel mimicking pancreatitis.[9] Obstruction occurs as a result of (i) luminal stenosis (due to mural thickening and smooth muscle hyperplasia), (ii) disruption of Auerbach’s plexus and the submucosal Meissner plexus and interstitial cells of Cajal and, (iii) inflammatory adhesions.[14]
In a recent study of 89 patients with BE from the Mayo Clinic, preoperative confirmation was shown to be uncommon; colonoscopy with biopsy confirmed the diagnosis in 29.6% and only 15% of patients had histological lesions involving mucosa.[15] Endoscopic biopsies usually yield insufficient tissue due to involvement of deep layers of the bowel wall.[16] On transvaginal or transrectal ultrasound, bowel nodules appear as irregular, hypoechoic masses penetrating into the intestinal wall.[17] Barium enema, CT/CT enteroclysis, magnetic resonance imaging (MRI), rectal endoscopic ultrasound all have a complementary role in preoperative diagnosis.[3] Multislice CT scan detects lesions outside the pelvis with an overall sensitivity of 95%.[18] Rectal endoscopic ultrasound is a newer modality that helps to identify nodules in the rectovaginal septum (sensitivity 96%, specificity 100%) and rectal infiltration (sensitivity 92%, specificity 66%).[19]
References
- Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–69.
- De Ceglie A, Bilardi C, Blanchi S, Picasso M, Muzio MD, Trimarchi A, et al. Acute small bowel obstruction caused by endometriosis: a case report and review. World J Gastroenterol. 2008;14:3430–4.
- Remorgida V, Ferrero S, Fulcheri E, Ragni N, Martin DC. Bowel endometriosis: presentation, diagnosis, and treatment. Obstet Gynecol Surv. 2007;62:461–70.
- Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. 2003;18:157–61.
- Paksoy M, Karabicak I, Ayan F, Aydogan F. Intestinal obstruction due to rectal endometriosis. Mt Sinai J Med. 2005;72:405–8.
- Orbuch IK, Reich H, Orbuch M, Orbuch L. Laparoscopic treatment of recurrent small bowel obstruction secondary to ileal endometriosis. J Minim Invasive Gynecol. 2007;14:113–5.
- Jubanyik KJ, Comite F. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1997;24:411–40.
- Anaf V, El Nakadi I, Simon P, Van de Stadt J, Fayt I, Simonart T, et al. Preferential infiltration of large bowel endometriosis along the nerves of the colon. Hum Reprod. 2004;19:996–1002.
- Wong LS, Mahendrakumar R, Bullen BR. Small-bowel endometriosis masquerading as pancreatitis. J R Soc Med. 1999;92:17–8.
- Slavin RE, Krum R, Van Dinh T. Endometriosis-associated intestinal tumors: a clinical and pathological study of 6 cases with a review of the literature. Hum Pathol. 2000;31:456–63.
- Bianchi A, Pulido L, Espín F, Hidalgo LA, Heredia A, Fantova MJ, et al. Intestinal endometriosis. Current Status. Cir Esp. 2007;81:170–6.
- de Bree E, Schoretsanitis G, Melissas J, Christodoulakis M, Tsiftsis D. Acute intestinal obstruction caused by endometriosis mimicking sigmoid carcinoma. Acta Gastroenterol Belg. 1998;61:376–8.
- Mussa FF, Younes Z, Tihan T, Lacy BE. Anasarca and small bowel obstruction secondary to endometriosis. J Clin Gastroenterol. 2001;32:167–71.
- Remorgida V, Ragni N, Ferrero S, Anserini P, Torelli P, Fulcheri E. The involvement of the interstitial Cajal cells and the enteric nervous system in bowel endometriosis. Hum Reprod. 2005;20:264–71.
- Kaufman LC, Smyrk TC, Levy MJ, Enders FT, Oxentenko AS. Symptomatic intestinal endometriosis requiring surgical resection: clinical presentation and preoperative diagnosis. Am Gastroenterol. 2011;106:1325–32.
- Garry R. Electrosurgical resection of endometriosis. In: Redwine DB (ed). Surgical Management of Endometriosis. London: Taylor & Francis; 2004:71–85.
- Bazot M, Darai E. Sonography and MR imaging for the assessment of deep pelvic endometriosis. J Minim Invasive Gynecol. 2005;12:178–85.
- Biscaldi E, Ferrero S, Fulcheri E, Ragni N, Remorgida V, Rollandi GA. Multislice CT enteroclysis in the diagnosis of bowel endometriosis. Eur Radiol. 2007;17:211–9.
- Roseau G, Dumontier I, Palazzo L, Chapron C, Dousset B, Chaussade S, et al. Rectosigmoid endometriosis: endoscopic ultrasound and clinical implications. Endoscopy. 2000;32:525–30.
|