Madhu Sasidharan,1 Srinivas Nistala,1 Narendhran RT,1 Murugesh M,1 Shobna J Bhatia,2 Pravin M Rathi1
Department of Gastroenterology and Hepatology,
TNMC–BYL Nair Hospital,1
Mumbai;
Department of Gastroenterology and
Hepatology,
KEM Hospital,2 Mumbai, India
Corresponding Author:
Dr. Pravin M Rathi
Email: rathipmpp@gmail.com
Abstract
Background and aims: Malnutrition is commonly associated with chronic liver disease. The presence of protein–calorie malnutrition has been shown to be associated with increased short- and long-term mortality in patients with acute and chronic liver disease. We undertook this study to assess the prognostic value of nutritional status in predicting survival in cirrhotic patients. The aim of our study was to determine whether assessment of nutritional status using the RFH-SGA score adds significantly to CP (Child-Pugh) and MELD scores in predicting patient prognosis and survival in cirrhotic patients.
Methods: Diagnosed cases of cirrhosis were enrolled and their nutritional assessment was done using the RFH-SGA score. All patients were followed up for a period of 6 months. The mortality rates in the various groups were compared with respect to their nutritional status. Multivariate analysis was used to determine the factors associated with mortality.
Results: A total of 73 cirrhotic patients were taken up for this study. Of these, 23 patients (31.5%) were well nourished, 21 (28.8%) had mild to moderate malnourishment and 29 (39.7%) were severely malnourished. Multivariate analyses of various parameters identified poor nutritional status, increased CP grade, increased creatinine, lower sodium levels and longer prothrombin time as being independently associated with poorer survival.
Conclusions : RFH-SGA is a simple and inexpensive tool for assessing the nutritional status in cirrhotic patients and can reliably predict their disease prognosis and survival.
|
48uep6bbphidvals|552 48uep6bbph|2000F98CTab_Articles|Fulltext Malnutrition and micronutrient deficiencies are common in patients with liver disease. The pathogenesis of protein-energy malnutrition in cirrhosis involves many factors, including poor oral intake, malabsorption, and metabolic abnormalities similar to that found in stress.
The Child–Pugh (CP) classification is a modification of the Child–Turcotte classification, which has been widely used as an index of disease severity for patients with end-stage liver disease since 1973.[1]Whilst the original Child–Turcotte classification included nutritional status, this was replaced with prothrombin time (PT) in the CP classification.[2] The presence of protein–calorie malnutrition has been shown to be associated with increased short- and long-term mortality in patients with acute and chronic liver disease.[3] In Alberino’s study,[4] malnutrition was shown to be an independent predictor of survival in which the inclusion of mid-arm muscle circumference (MAMC) and triceps skin fold thickness (TST) improved the prognostic accuracy of the CP score. Thus it is likely that nutritional status could be a useful addition to the CP classification when assessing the prognosis of cirrhotic patients. Abbott et al[5] investigated the relationship between the CP classification and nutritional indicators and found that advanced CP classification was associated with diminished muscle status and greater early postoperative morbidity after liver transplantation.
Similar to the CP classification, MELD does not incorporate any measures of nutritional status. The identification of an optimal method of nutritional assessment in patients with cirrhosis is difficult because many of the traditionally measured parameters of nutritional status, such as weight, energy panels and biochemical values vary with the severity of liver disease independent of the patient’s nutritional status.[5] Subjective Global Assessment (SGA) uses clinical criteria to determine nutritional status without the use of objective measurements.[6]
It is more useful than objective measures alone for identifying individuals at nutritional risk because of an ability to encompass a multitude of factors influencing nutritional status. The SGA has been validated in liver transplant candidates.[7]
Therefore the goal of this study was to evaluate the prognostic value of adding nutritional status to either the CP or MELD classification while assessing the prognosis of cirrhotic patients.
 Methods
This was a prospective follow-up study for 6 months. Ethics committee approval was obtained before starting the study. All consecutive cirrhotic patients who were admitted or seen in the out-patient department at our institution were enrolled after informed consent. The primary endpoint of this study was all-cause mortality. For the analyses reported in this paper, patient follow-up is considered from the date of assessment until the date of death or last follow-up visit, as applicable. A Cox proportional hazards regression model was employed for statistical analysis. Factors associated with survival in univariate analysis were identified using the regression model. This approach considers outcome over each patient’s entire follow-up, rather than arbitrarily classifying patients at a single time point (e.g. 3 months, 1 year), as is often done in logistic regression analysis. All factors significantly associated with survival (p <0.1) in univariate analyses were included in a multivariable model using a two-stage selection procedure. Initially, demographic and liver-related parameters were included in the model using a backwards selection procedure to identify factors independently associated with survival. Once the final model had been selected each nutritional parameter was tested to see whether its inclusion significantly improved the fit of the model. All analyses were performed using the PHREG procedure in the statistical software package SPSS.
Methods
This was a prospective follow-up study for 6 months. Ethics committee approval was obtained before starting the study. All consecutive cirrhotic patients who were admitted or seen in the out-patient department at our institution were enrolled after informed consent. The primary endpoint of this study was all-cause mortality. For the analyses reported in this paper, patient follow-up is considered from the date of assessment until the date of death or last follow-up visit, as applicable. A Cox proportional hazards regression model was employed for statistical analysis. Factors associated with survival in univariate analysis were identified using the regression model. This approach considers outcome over each patient’s entire follow-up, rather than arbitrarily classifying patients at a single time point (e.g. 3 months, 1 year), as is often done in logistic regression analysis. All factors significantly associated with survival (p <0.1) in univariate analyses were included in a multivariable model using a two-stage selection procedure. Initially, demographic and liver-related parameters were included in the model using a backwards selection procedure to identify factors independently associated with survival. Once the final model had been selected each nutritional parameter was tested to see whether its inclusion significantly improved the fit of the model. All analyses were performed using the PHREG procedure in the statistical software package SPSS.
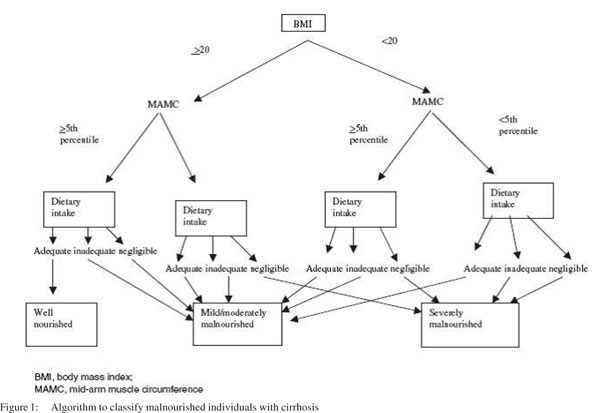
Demographic factors considered were age, gender and etiology of disease. Severity of liver disease was assessed using bilirubin, albumin, INR, PT, CP score, urea, creatinine, ascites (none, slight or moderate), encephalopathy (none/grade 1–2/grade 3–4), and CP classification (A/B/C). Nutritional parameters evaluated were weight, dry weight, height (estimated), body mass index (BMI),mid upper arm circumference( MAC), MAMC, TST and Royal Free Hospital – Subjective Global Assessment (RFH-SGA)[well nourished/ mild or moderately malnourished/severely malnourished] (Figure 1).
We used Kaplan–Meier and Cox proportional hazards regression models to prospectively identify factors associated with mortality in a cohort of 73 cirrhotics [M/F:61/12; median age: 42 years (12-62)] with prospectively collected nutritional parameters as well as modified subjective global nutritional assessment, Royal Free Hospital-Subjective Global Assessment index.Apart from nutritional parameters other variables were similar to those in Child–Pugh and Model for End-Stage Liver Disease scores, including age, etiology of cirrhosis and renal function. The etiology of liver disease was alcoholism in 55 patients, chronic hepatitis B (CHB) in 6, autoimmune cirrhosis in 4, and Wilson’s disease in 5. All patients underwent a systematic work-up on admission, all data were collected prospectively. Clinical variables recorded included degree of encephalopathy (none, grade 1–2, grade 3–4), and degree of ascites (absent, slight or moderate), based on clinical or ultrasound data. The laboratory data collected included bilirubin, albumin, PT, international normalized ratio (INR), urea and creatinine, which were used to calculate the CP and MELD scores when the patients were haemodynamically stable. All markers were measured by established standard laboratory methods.
Recent dietary intake was assessed using an established diet history method8 supplemented, where necessary, with additional information from relatives, nursing staff and food record sheets. Details of dietary restrictions and oral, enteral or parenteral nutritional support were recorded. The data obtained provided a quantitative evaluation of the overall adequacy of the diet in relation to estimated requirements. Intakes were categorized as adequate if they met estimated requirements, inadequate if they failed to meet estimated requirements but exceeded 500 kcal/day, or negligible if they provided <500 kcal daily. Nutritional assessment included dry weight [estimated by subtracting paracentesis (total) volume, 1 L = 1 k],9 BMI, anthropometric indices (TST,MAC and MAMC), body fat, weight change, dietary intake, the use of a dietary supplement and the RFH-SGA. Reference values were derived from the Bishop’s study10 according to age and gender. An RFH-SGA index adds BMI, TST and MAMC to the standard SGA, which are all objectively measurable and reproducible variables. As per this index patients were classified prospectively into three groups: well nourished, mild or moderately malnourished, and severely malnourished, similar to the classification by standard SGA. Other factors such as recent weight loss or severe steatorrhoea, which might have additional effects on nutritional status, were taken into account by incorporating a subjective override which allows the assessor to change the nutritional class of a patient only by a single category within the three described above.[7,11] The CP classification was employed as outlined by Pugh et al[2] and the MELD score was calculated as 6.43 + 9.57 · loge (creatinine) + 3.75 · loge (bilirubin) + 11.2 · loge (INR) and was rounded to the nearest integer.[12,13] Nutritional and other laboratory parameters of the patients who survived were compared with those of the patients who died.
Results
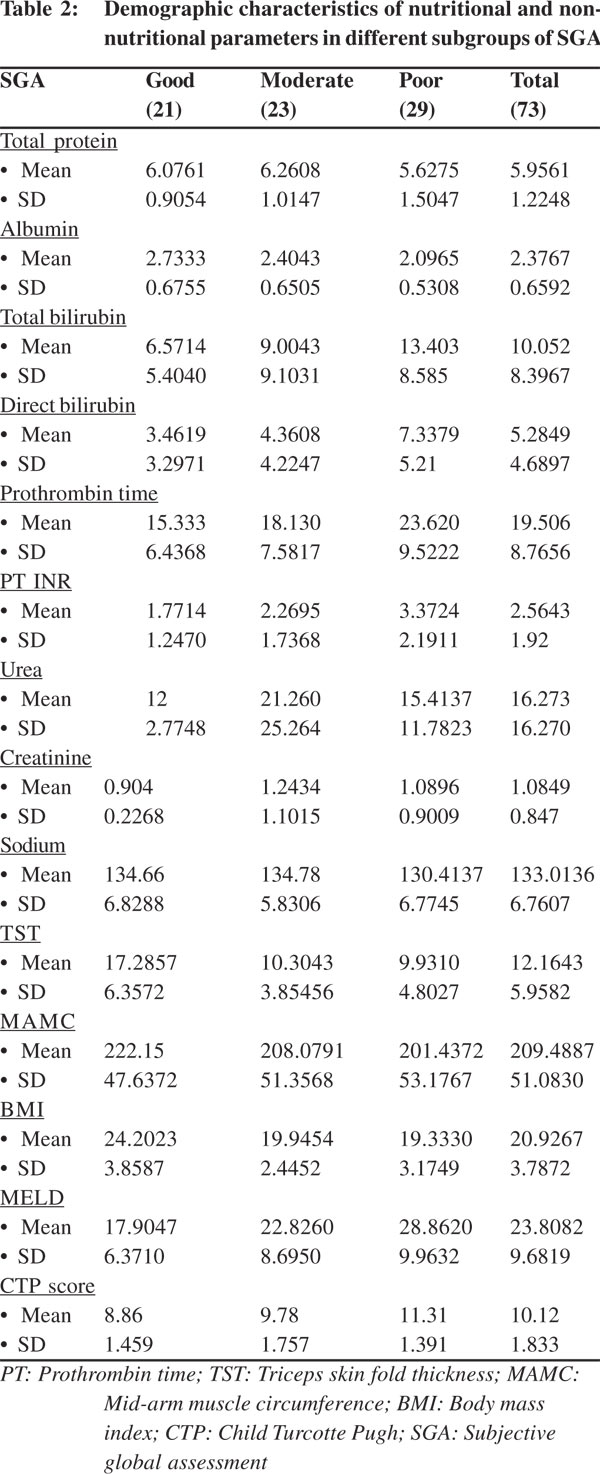
Seventy three patients were enrolled in this study and were followed-up for a period of minimum 6 months. Majority of the patients were males. The etiology of liver disease was alcoholism in 55, chronic hepatitis B (CHB) in 6, autoimmune cirrhosis in 4, and Wilson’s disease in 5. Out of the total 73 patients studied, 23 patients (31.5%) were well nourished, 21 (28.8%) were mild or moderately malnourished and 29 (39.7%) were severely malnourished, according to the RFH-SGA scale . BMI was more than 20 in 63% of the patients . The Child Pugh score revealed that majority of patients i.e. 43 (58.9%), belonged to Child C group and 29 patients (39.73%) to Child B with only one patient in the Child A group.
 Individuals who were severely malnourished were more likely to have moderate ascites (p=0.0002) and higher CP scores (p=0.007) compared with those who were well nourished or only mild/moderately malnourished. Those with mild/moderate malnourishment were most likely to have grade 1–2 encephalopathy (p=0.02), but there were no significant differences between the groups with respect to any other clinical parameters (p >0.05 in each case). As expected, global nutritional status was significantly associated with all other nutritional parameters measured (p=0.0001 for all variables), with the exception of height (p=0.82). Fifty three patients were alive at the last follow-up without transplantation and 42 of them had alcoholic liver disease with nearly 50% of them under abstinence during the follow-up.
Individuals who were severely malnourished were more likely to have moderate ascites (p=0.0002) and higher CP scores (p=0.007) compared with those who were well nourished or only mild/moderately malnourished. Those with mild/moderate malnourishment were most likely to have grade 1–2 encephalopathy (p=0.02), but there were no significant differences between the groups with respect to any other clinical parameters (p >0.05 in each case). As expected, global nutritional status was significantly associated with all other nutritional parameters measured (p=0.0001 for all variables), with the exception of height (p=0.82). Fifty three patients were alive at the last follow-up without transplantation and 42 of them had alcoholic liver disease with nearly 50% of them under abstinence during the follow-up.
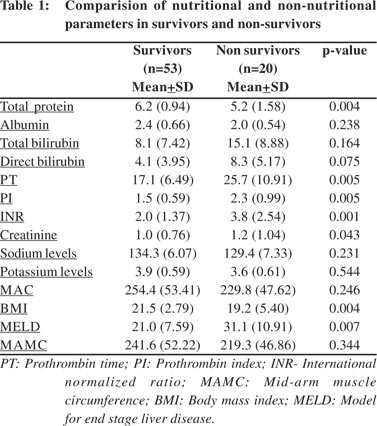
In univariate analysis of non-nutritional parameters, lower total protein (p=0.004), higher INR (p=0.001), increased PT/PI (p=0.005), lower BMI (p=0.004), higher MELD (p=0.007) and higher creatinine levels (p=0.043) were found associated with shorter survival (Tables 1 & 2). In addition, the presence of slight to moderate ascites or grade 3–4 encephalopathy was also associated with shorter survival. The CP score (p=0.0001) and the CP grade (p=0.0002) were associated with shorter survival, but a history of bleeding varices and etiology of liver disease were not significantly associated with the shorter survival.
Multivariate analysis revealed increased CP grade,increased creatinine levels, lowered sodium levels and longer PT to be independently associated with poorer survival. Although creatinine levels were found significantly associated with survival, they were not included in the final model because of measurement variability in presence of high bilirubin. The results from models that included the CP score rather than the grade were found similar.
 Comparing the various parameters between patients who survived versus those who succumbed to their disease at 6 month follow-up revealed significant associations with total protein, INR , and creatinine levels, and the patient’s BMI and MELD scores (Table 1 & 2). One patient succumbed in both the well nourished and mild/moderately malnourished SGA groups. The death in well nourished group occurred in third month of follow-up and while that in the mild/moderately malnourished group occurred in first month itself. There were 18 deaths among the 29 patients who were grouped under the severely malnourished group as per the RFH-SGA score. Out of these, nine deaths occurred in the first month of follow-up, three in the second month, four in the third month and one death each in months four and seven after enrollment.
Comparing the various parameters between patients who survived versus those who succumbed to their disease at 6 month follow-up revealed significant associations with total protein, INR , and creatinine levels, and the patient’s BMI and MELD scores (Table 1 & 2). One patient succumbed in both the well nourished and mild/moderately malnourished SGA groups. The death in well nourished group occurred in third month of follow-up and while that in the mild/moderately malnourished group occurred in first month itself. There were 18 deaths among the 29 patients who were grouped under the severely malnourished group as per the RFH-SGA score. Out of these, nine deaths occurred in the first month of follow-up, three in the second month, four in the third month and one death each in months four and seven after enrollment.
Discussion
Our results revealed that the RFH-SGA is a simple and inexpensive tool for assessing the nutritional status in cirrhotic patients and can reliably predict their disease prognosis and survival. Patients with poor nutritional status are more likely have poor survival as compared to their better nourished counterparts, irrespective of their Child or MELD scores. Nutrition assessment by a validated protocol[7,11] provided additional prognostic information on cirrhotic patients beyond that provided by routine clinical indicators (including CP grade, urea and PT). Specific nutrition variables lacking correlation with malnutrition in cirrhotic patients,[14,15] did not significantly enhance the prognosis provided by the liver parameters, but were captured by SGA which is really a ‘nutritional review’ and has been shown to be more useful in identifying patients at risk of malnutrition.[6,16]
The assessment of this parameter is as subjective as that of ascites and encephalopathy. It is clinically recognized that malnutrition is important for prognosis of cirrhotic patients.[3,4] SGA and serum proteins correlated with the severity of liver disease. Handgrip strength and MAMC were crucial nutritional parameters in detecting body cell mass depletion that has been associated with adverse outcomes in patients with end-stage liver disease,[3,4] with handgrip strength being a better predictor than standard SGA or the prognostic nutritional index. In this study, patients with a poor RFH-SGA score suffered higher mortality compared to patients with good nutrition to only mild-moderate malnourishment. The CTP and MELD scores gradually increased from the well nourished group to severely malnourished group, correlating well with the RFHSGA scores. The RFH-SGA score was significantly poorer in patients who died than those who survived. Patients with a poor nutritional status had a worse prognosis compared to patients with good nutrition even if their CTP and MELD scores were similar. Addition of nutritional parameters to the Child and MELD scores could predict mortality better than either alone.
Gunsar et al[17] prospectively analysed 222 cirrhotics based on dry weight, anthropometric indices and previously validated subjective global assessment (SGA) of nutrition, with concomitant scoring of CP’s parameters, age, etiology of cirrhosis and renal function with respect to survival. Multivariate models showed that MELD was not superior to CTP score. SGA was found significantly associated (p=0.0006) with mortality, and SGA improved the CTP model. The most useful model had the relative hazards of creatinine 4.86 (p=0.004), SGA 2.8 (p=0.0006), age 1.61 (p=0.0001), CTP score 1.27 (p=0.01) and PT 1.08 (p=0.004). Thus nutritional indices as well as renal function add significantly to the CTP score. This study suggests that important prognostic information is contained in the nutritional state and renal function of cirrhotics over and above the standard CTP and MELD scores. Mohandas et al18 analysed SGA scores in 294 patients undergoing cancer surgery and found that SGA scoring is a simple and inexpensive method for assessing malnutrition in cancer patients undergoing surgery. The length of postoperative stay and the number of antibiotic days revealed a significant trend from SGA-A to SGA-C (p=0.000). Predefined adverse events occurred in 7.9%, 17.3% and 25% of SGA groups A, B, and C, respectively (p=0.025). The risk for adverse events was significantly higher in SGA-C group (OR 5.27, 95% CI 1.35-20.51, p<0.016) compared to SGA-A group. Low BMI was not associated with postoperative adverse outcomes and its use for nutritional screening is likely to overestimate severe malnutrition in Indian patients. They concluded that the SGA rather than the BMI predicted the postoperative outcomes better. In a study by Sarin et al[19] assessing dietary and nutritional abnormalities in alcoholic liver disease, protein energy malnutrition was common in both alcoholic and nonalcoholic cirrhotics, but was more pronounced in the latter. The degree and profile of malnutrition in chronic alcoholics and in alcoholic cirrhotics were comparable.
It is still not clear, whether MELD is better than CTP score for predicting survival in patients with chronic liver disease outside of liver transplant waiting lists, or whether its superiority is mainly related to the use of creatinine in the MELD score. This is surprising as CTP score is an ‘empirical score’ and has never been validated statistically. Heuman et al[20] recently reexamined statistically the traditional cut-off points of CTP classification (A, B and C). They found that these divisions were suboptimal for short-term prognosis in 599 cirrhotics referred for LT and they proposed new CTP subclasses: A (5- 6), B1 (7-8), B2/C1 (9-11), C2 (12-13) and C4 (14-15). In the same study[17] traditional CTP and MELD scores had similar discriminative ability for short- and long-term survival. Another proposal is to evaluate CTP score with the addition of creatinine and perhaps nutritional status, a factor in the original Child score, both of which have well-known parameters which influence prognosis in cirrhotic patients. An indicator of portal hypertension (presence or size of varices, previous bleeding, hepatic venous pressure gradient) might also be added in an effort to ameliorate prognostic capacity of CTP score without losing its advantages.
Abbott et al[5] found that a reduced muscle mass was associated with the advanced CP classification and an increased and earlier post-OLT morbidity. The addition of MAMC and TSF to the CP score in cirrhosis improved its prognostic accuracy in one study.[21] One study reported that Shaw’s risk score (later contained a nutrition assessment) did not influence survival at 6 months after liver transplantation.[22] Another had a weak but statistically significant correlation between death and MAC after liver transplantation.[23] Although CP classification has been the most widely used tool for prognosis in cirrhotic patients, it uses discrete cut-offs to move from one class to the next resulting in limited discriminatory ability.[1,2,12,23] Even when the CP score, as opposed to CP grade, is used, there are only 10 different points between the least sick (CP score = 5) and the most advanced (CP score =15) patients.2 In contrast, the MELD score that uses continuous measurements of variables may provide better discrimination of mortality over a 3-month period.[12,23] In this study, both urea and creatinine, known markers of renal function, also added prognostic power to the CP classification and RFH-SGA. In a study by Papatheodoridis[24] creatinine-modified CP was found better than CP alone but creatinine-modified CP was similar to MELD for predicting survival in patients with decompensated cirrhosis. Renal function is a well recognized predictor of survival in patients with liver disease. Nair et al[25] found that creatinine clearance <40 mL/min at the time of transplant was associated with significantly lower short and long term graft and patient survival rate. Whilst the MELD score also incorporates serum creatinine level as a parameter of disease severity index for patients with chronic liver diseases, we found that urea levels were also independently associated with outcome in analyses that incorporated the MELD score rather than CP grade. This study therefore confirms the importance of renal function for the prediction of survival of patients with chronic liver disease.
There are some limitations that should be noted in this study. Firstly, the RFH-SGA contains a subjective measurement and its assessment, as with ascites and encephalopathy, may differ amongst individual clinicians or centers. Nevertheless, the recognition of severe malnutrition vs. other states by the standard SGA does have reasonable discrimination and has been validated in liver transplant candidates[16,7] which is the cohort in which nutrition is usually assessed as a routine. Thus, despite the subjective nature part of the RFH-SGA index, this study does provide evidence for nutrition as an independent prognostic parameter in late stage cirrhosis and should stimulate therapeutic studies in this area, including its significance for survival after transplantation. These studies would also be needed to test the universal applicability of the RFH-SGA index when compared with the standard SGA index,[16,7] although the added parameters of BMI, TST and MAMC (RFH-SGA) are not subjective measurements. In addition, as MAMC is a product of MAC and skin thickness, there may be little to gain by using both TST and MAMC, but both were included in the original published RFH-SGA index[21,22] which we used unchanged.
The second limitation is that the RFH-SGA index achieved statistical significance in the multivariate analysis only in the severely malnourished group and not in the less severely malnourished group, in which other variables appeared to have sufficient prognostic value. However, the significant association in the univariate analysis would suggest that statistical effect is related to sample size, but this will need evaluation in a larger cohort and it could be that with new reference values for the general population (those in Bishop’s study[10] are from 25 years ago), all the cirrhotics would appear more malnourished.
The third limitation is that our cohort was not sufficiently large to randomly split into a training sample and a test sample. Thus our model has not been validated in this paper, but the fact that both the models censoring at the time of transplantation and competing risks model yield similar results makes it robust. Nevertheless, validation of these findings is particularly important, as the value of the RFH-SGA index may vary from centre to centre. Thus we encourage other authors to validate our findings in their own patient groups, particularly where these patients are in different settings. The modified SGA was neither revalidated nor checked for inter-observer agreement before applying in this study. The SGA tool has been evaluated in different patient populations around the world and has been used for over 20 years and has produced consistent results.
In conclusion, individuals who were severely malnourished were more likely to have moderate ascites and higher CP scores compared to those who were well nourished or only mild/ moderately malnourished. Those with mild/moderate malnourishment were most likely to have grades 1–2 encephalopathy (p=0.02). In univariate analysis of the nonnutritional parameters, lower total protein, higher INR, increased PT/PI, lower BMI, higher MELD and higher creatinine were all associated with shorter survival. In addition the presence of slight to moderate ascites and grade 3–4 encephalopathy were also associated with shorter survival. History of bleeding varices and etiology of liver disease were not significantly associated with shorter survival. In multivariate analysis of the non-nutritional parameters, increased CP grade, increased creatinine, lower sodium levels and longer PT were independently associated with poorer survival .
References
- Child C, Turcotte J. The liver and portal hypertension. In: Child CI, ed. Surgery and Portal Hypertension. Philadelphia, USA: W. B. Saunders, 1964:50–8.
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC and Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9.
- Alveras-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment,and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–7.
- Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445–50.
- Abbott WJ, Thomson A, Steadman C, Gatton ML, Bothwell C, Kerlin P, et al. Child-Pugh class, nutritional indicators and early liver transplant outcomes. Hepatogastroenterology. 2001;48:823–7.
- Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13.
- Stephenson GR, Moretti EW, El-Moalem H, Clavien PA and Tuttle-Newhall JE. Malnutrition in liver transplant patients: preoperative subjective global assessment is predictive of outcome after liver transplantation. Transplantation. 2001;72:666–70.
- Morgan MY, Madden AM. Aspects nutritionnels des hepatopathies croniques stables. Nutr Clin Metabol. 1999;13:246–54.
- Inadomi J, Cello JP and Koch J. Ultrasonographic determination of ascitic volume. Hepatology. 1996;24:549–51.
- Bishop CW, Bowen PE and Ritchey SJ. Norms for nutritional assessment of American adults by upper arm anthropometry. Am J Clin Nutr. 1981;34:2530–9.
- Morgan MY, Madden AM. The derivation, validation and clinical application of a new global method for assessingnutritional status in cirrhotic patients. Hepatology. 1999;30:4 (abstract).
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70.
- Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J and ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71.
- Blackburn GL, Bistrian BR, Maini BS, Schlamm HT and Smith MF. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr. 1977;1:11–22.
- Pieterse S, Manandhar M and Ismail S. The association between nutritional status and handgrip strength in older Rwandan refugees. Eur J Clin Nutr. 2002;56:933–9.
- Hasse J, Strong S, Gorman MA and Liepa G. Subjective global assessment: alternative nutrition-assessment technique for livertransplant candidates. Nutrition. 1993;9:339–43.
- Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563–72.
- Shirodkar M and Mohandas KM. Subjective global assessment: a simple and reliable screening tool for malnutrition among Indians. Indian J Gastroenterol. 2005;24:246–50.
- Sarin SK, Dhingra N, Bansal A, Malhotra S and Guptan RC. Dietary and nutritional abnormalities in alcoholic liver disease: a comparison with chronic alcoholics without liver disease. Am J Gastroenterol. 1997;92:777–83.
- Heuman DM, Habib A, Abou-Assi S, Williams L, Mihas A. Rationally derived Child-Turcotte-Pugh (CTP) subclasses permit accurate stratification of near-term risk in cirrhotics patients referred for liver transplantation. Gastroenterology. 2005;128:A–734.
- Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, et al. Impact of nutritional status on outcomes after liver transplantation. Transplantation. 2000;70:1347–52.
- Deschenes M, Villeneuve JP, Dagenais M, Fenyves D, Lapointe R, Pomier-Layrargues G, et al. Lack of relationship between preoperative measures of the severity of cirrhosis and shortterm survival after liver transplantation. Liver Transpl Surg. 1997;3:532–7.
- Shahid M, Johnson J, Nightingale P and Neuberger J. Nutritional markers in liver allograft recipients. Transplantation. 2005;79:359–62.
- Papatheodoridis GV, Cholongitas E, Dimitriadou E, Touloumi G, Sevastianos V and Archimandritis AJ. MELD vs Child-Pugh and creatinine-modified Child-Pugh score for predicting survival in patients with decompensated cirrhosis. World J Gastroenterol.
- Nair S, Verma S and Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–85.
|