Lileswar Kaman,1 Javid Iqbal,1 Mahander Pall,1 Ishwar Bhukal,2 Arunanshu Behera,1 Gurpreet Singh,1 Rajinder Singh1
Departments of General Surgery1
and Anesthesia,2
Postgraduate Institute of Medical
Education and Research,
Chandigarh, India
Corresponding Author:
Dr. Lileswar Kaman
Email: kamanlil@yahoo.com
Abstract
Background: Although pancreatic trauma is uncommon, it poses a diagnostic and therapeutic challenge. Any delay in diagnosis raises morbidity and mortality. This study highlights the current management and outcome in patients of pancreatic trauma at a single tertiary care center.
Methods: This is a retrospective analysis of prospectively collected data of 24 patients diagnosed to have pancreatic trauma. Collected data was analyzed for age, gender, mechanism of injury, hemodynamic status at presentation, initial serum amylase levels, CECT abdomen findings, AAST-OIS grade of pancreatic injury, injury to other organs, management, complications and outcome.
Results: The mean age of these 24 patients was 25 years; 19 were male and 5 females. The mechanisms of pancreatic trauma included blunt abdominal trauma in 21 (87.5%) cases and penetrating injury in 3 (12.5%). Seven (29.16%) patients were managed by non-operative management and 17 (70.83%) underwent surgery. Complications were more frequent in the operative group as compared to the non-operative group. Neither endocrine deficiency nor any mortality was noted in the non-operative management group; while there were 2 cases of endocrine deficiency and 3 mortalities in the operative group.
Conclusions: Pancreatic trauma is more common in young male patients and more commonly inflicted by motor vehicles accidents. Low grade blunt pancreatic injury in hemodynamically stable patients and selected patients with high grade blunt pancreatic injury can be managed successfully by non-operative management with no increase in morbidity or mortality and most patients with high grade blunt pancreatic injury and those having penetrating injuries need surgical intervention.
|
48uep6bbphidvals|534 48uep6bbph|2000F98CTab_Articles|Fulltext In comparison to analogous injuries to other visceral organs, blunt trauma of the pancreas is less common because of its retroperitoneal location and subtle clinical presentation, frequently resulting in delayed diagnosis and treatment. Pancreatic injury secondary to trauma is uncommon but carries significant morbidity and mortality. Pancreatic injury occurs in 0.2% of patients with blunt trauma abdomen.[1] The incidence is higher in penetrating injuries, ranging from 1 to 12% in published series.[1,2] The mortality directly attributed to pancreatic injury ranges from 2 to 17%.[3] Injuries to the pancreas have been associated with reported morbidity rates approaching 45%.[2-6] If treatment is delayed, these rates may increase to 60%.[4-6] Integrity of the main pancreatic duct is the most important determinant of outcome after injury to the pancreas.[4] If the pancreas is otherwise normal, a resection of more than 80% can be done without endocrine deficiency.[3]
Methods
This was a retrospective analysis of prospectively collected data of patients diagnosed with pancreatic trauma and admitted to a tertiary referral health centre in north India from January 2007 to November 2009. The collected data was analyzed for age, gender, mechanism of injury, hemodynamic status at presentation, initial serum amylase levels, contrast enhanced computed tomography (CECT) imaging study of the abdomen, American Association for the Surgeon of Trauma - Organ Injury Scale (AAST-OIS) grade of pancreatic injury (Table 1), injury to other organs, management, complications and outcome.
Results
Total 24 patients were included in the study; among them 19 were male and 5 were female. Patient age ranged between 13 to 48 years with a mean of 25 years. Out of the 24 patients diagnosed with pancreatic trauma, 21 (87.5%) had blunt trauma abdomen (BTA) and 3 (12.5%) had penetrating injury abdomen.
Among blunt trauma abdomen patients 9 met with an accident while driving a two wheeler, 5 had steering wheel injury, 3 had BTA during a pillion ride, 2 were injured by assault, 1 had bull gore injury and 1 was injured by collapse of a wall on the patient. All penetrating pancreatic injuries were due to stab injury abdomen. The diagnosis was suspected on the basis of nature of injury (blunt/penetrating, seat belt injury, cycle handlebar injury), site of injury (central abdomen), raised serum amylase levels and abdominal signs. Excluding 3 patients who presented with perforation peritonitis, the diagnosis of pancreatic injury was not considered in the initial examination in 4 out of the remaining 21 patients. Subsequent assessment of raised serum amylase levels and disproportionate abdominal signs on clinical examinations lead to the possible diagnosis of pancreatic injury and CECT eventually confirmed the diagnosis. Out of these 4 patients where the diagnosis of pancreatic injury was not considered initially, 3 had grade II and 1 patient had grade III injury.
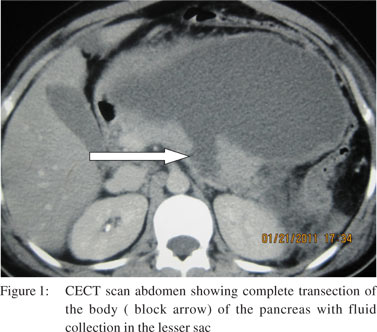
The hemoglobin of patients ranged from 7.2 to 15.9 gm% with a mean value of 12.2 grams% and one patient required pre-operative blood transfusion. Serum amylase values of various patients ranged from 102 to 3250 IU at admission, with a mean of 1084.08 IU. We collected all blood samples for measuring serum amylase levels at least 3 hours after trauma and it was raised in all patients. All patients underwent CECT abdomen (Figure 1) except 3 patients who presented with signs of peritonitis. In addition to the diagnosis of pancreatic trauma, 11 patients were diagnosed to have associated solid organ injury on CECT abdomen. Among them 8 had liver trauma, 5 had splenic trauma and 4 had renal trauma. No complex surgical procedure was performed for liver trauma except for hemostasis in 5 patients, 4 patients needed splenectomy along with distal pancreatectomy, 1 patient underwent splenectomy for grade IV splenic injury with no pancreatic resection and 1 patient needed left nephrectomy for grade V renal injury. Three patients were diagnosed to have blunt trauma chest and 2 patients were diagnosed to have head injury and were managed by the concerned specialist teams.
 Twenty one patients underwent CECT abdomen and these patients were divided into three groups on the basis of severity of pancreatic injury: i) mild (grades I and II): 11(45.83%); ii) moderate (grade III): 6 (25%); iii) severe (grades IV and V): 4 (16.66%).
Management
Non-operative management
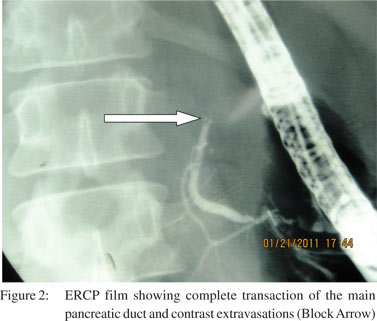
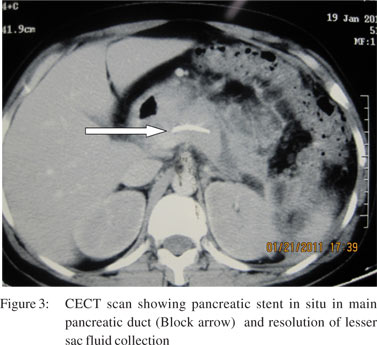
Seven patients were managed by non-operative management. All these patients were hemodynamically stable and had no other indications for laparotomy. Among these patients, five were managed conservatively on a nil oral regimen, intravenous fluids, analgesics and intravenous antibiotics and two patients were managed by endoscopic retrograde cholangiopancreatography (ERCP) and pancreatic duct stenting (Figures 2 and 3). One patient had grade III and one had grade IV pancreatic injury. Three patients required pancreatic and one biliary stenting for post-operative management of pancreatic and biliary fistula. In this group two patients developed complications but there was no endocrine deficiency or mortality in this group.
Twenty one patients underwent CECT abdomen and these patients were divided into three groups on the basis of severity of pancreatic injury: i) mild (grades I and II): 11(45.83%); ii) moderate (grade III): 6 (25%); iii) severe (grades IV and V): 4 (16.66%).
Management
Non-operative management
Seven patients were managed by non-operative management. All these patients were hemodynamically stable and had no other indications for laparotomy. Among these patients, five were managed conservatively on a nil oral regimen, intravenous fluids, analgesics and intravenous antibiotics and two patients were managed by endoscopic retrograde cholangiopancreatography (ERCP) and pancreatic duct stenting (Figures 2 and 3). One patient had grade III and one had grade IV pancreatic injury. Three patients required pancreatic and one biliary stenting for post-operative management of pancreatic and biliary fistula. In this group two patients developed complications but there was no endocrine deficiency or mortality in this group.
Operative management
Out of 24 patients, 17 (70.83%) patients were managed by surgery. In this group, different pancreas-specific surgeries were performed depending on intra-operative findings and CECT abdomen findings. These included pancreatic debridement and lesser sac drainage (LSD) in six patients, omental pancreaticorraphy in four, only assessment and LSD in two, pancreatic necrosectomy and LSD in one, and distal pancreatectomy and closed suction drainage in four patients.
Three patients underwent surgery for management of complications: one underwent cystogastrostomy for pancreatic pseudocyst, second underwent end ileostomy and distal mucus fistula for enterocutaneous fistula and the third underwent spleen preserving distal pancreatectomy for pancreatic fistula which failed to close on conservative and endoscopic management. Among the operative group, seven patients were treated for gut perforation, two for segmental colonic necrosis along with pancreatic surgery in the same sitting. In the operative management group 13 patients developed complications, two cases suffered endocrine insufficiency and three succumbed to their condition.
A total of 13 patients had isolated pancreatic injury. Of these seven patients were managed non-operatively and six patients underwent pancreas-specific surgery. Out of the seven patients managed non-operatively, four patients had grade II injury. One patient each had grade I and grade III injuries. One patient had grade IV injury, which was managed successfully by ERCP and stenting. There was no mortality in this group. Out of the six patients who were operated, four patients had grade III injury, one patient had grade II and another patient had grade IV injury. Various pancreas-specific surgeries were performed. One grade IV injury patient died in the postoperative period.
Complications
Most common complication in our study was lung infection followed by pancreatic fistulae and intra-abdominal collections.
Five patients (20.83%) had pancreatic fistula in the postoperative period. Two were managed conservatively, and two by ERCP and pancreatic duct stenting and in the fifth patient the fistula failed to heal by ERCP and pancreatic ductal stenting and the patient had to undergo spleen preserving distal pancreatectomy. One patient (4.16%) developed ileal perforation and enterocutaneous fistula which was managed by laparotomy, end ileostomy and distal mucus fistula. One patient (4.16%) developed biliary fistula due to CBD injury and was managed by ERCP and NBD followed by stenting. Nine patients (37.50%) had hospital acquired/ ventilator associated pneumonia, and three among them developed adult respiratory distress syndrome (ARDS). All were treated by intravenous antibiotics, supportive treatment and ventilator support. Four patients (16.66%) had intra abdominal collections, out of which three were managed by ultrasound (USG) guided drainage and one by CT guided drainage. One patient (4.16%) experienced bleeding from the lesser sac drain which required laparotomy and hemostasis. One patient (4.16%) developed pancreatic pseudocyst and was managed by cystogastrostomy.
Outcome
Three patients (12.50%) died and two patients (8.33%) developed diabetes mellitus during follow-up. All these patients were from the operative group. Among mortalities, one patient developed bleeding from the lesser sac in the postoperative period; which needed re-exploration and later on developed enterocutaneous fistula for which he was operated on but the patient eventually succumbed to sepsis. Two patients presented late after trauma; both had gut perforations and succumbed to sepsis, adult respiratory distress syndrome (ARDS) and multi-organ dysfunction syndrome (MODS) in the post-operative period.
Discussion
Due to retroperitoneal location of the pancreas, the diagnosis of pancreatic injury can be a difficult and a challenging task even for the most experienced surgeon. Reasons for this include difficulty in diagnosing pancreatic injury on clinical examination because of lack of specific symptoms which even if present may be minimal, no reliable serum markers and underestimation of severity of injury on abdominal CT scan findings, especially in the first 24 hours after trauma.[7] Pancreatic trauma may be due to blunt abdominal trauma or penetrating injury abdomen. Blunt pancreatic injury occurs when high-energy force is applied to the upper abdomen, crushing the retroperitoneal structures against the vertebral bodies and causing a spectrum of injury from minor contusion to complete transection.[3,8] In adults, about 60% of pancreatic injuries are caused by motor vehicle accidents and consequent impact with the steering wheel, whereas in children the most common mechanism is adirect blow to the epigastrium from bicycle handlebars.[3] In our study only 20.8% patients had steering wheel injury and pancreatic injuries in most other patients were due to motor bike accidents, as most people in this region use motor bikes in view of better affordability as compared to cars. In a stab wound, the weapon damages the pancreatic tissue along the tract of the injury, and in a gunshot wound, the passage of the missile and the associated pressure wave causes a wider area of injury.8 Laparotomy is usually required because of evidence of major intraperitoneal bleeding or peritonitis. In blunt trauma, in the absence of indications for laparotomy, a high index of suspicion is required for diagnosis of pancreatic trauma.[3] The diagnosis of pancreatic injury should be made intra-operatively in all patients undergoing surgery.[9-11] Serum amylase was initially considered a reliable indicator of pancreatic trauma but further studies demonstrated that serum amylase is neither sufficiently sensitive nor specific to be used alone for the diagnosis of pancreatic injury.[12] In patients with blunt pancreatic trauma, 65–75% will manifest an elevated serum amylase. This number rises to 84% once 3 hours have elapsed between the injury and time of measurement.[3,13] Takishima et al[7] observed that the presence of hyper-amylasaemia after blunt pancreatic trauma is time dependent. Elevated serum amylase was present in all their cases when the samples were collected more than 3 hours following injury. The investigators concluded that serum amylase levels evaluated before this time period were not diagnostic. It has also been demonstrated that there is no relation between the degree of pancreatic trauma and the level of hyperamylasaemia.[7] In our study all samples were collected more than 3 hours after trauma and serum amylase level was raised in all patients. Wisner et al[14] reported that one third of patients with injuries as severe as complete pancreatic transection had serum amylase concentrations within the normal range. It is important to mention that raised serum amylase is not a reliable indicator of pancreatic injury in cases of brain injury, as significant percentage of these patients have hyperamylasaemia in the absence of abdominal trauma, suggesting that a central nervous system pathway is involved in the regulation of serum amylase levels.[15] In our study, the Pancreatic trauma diagnosis of pancreatic injury was not based only on serum amylase level, rather, all patients except those with peritonitis underwent immediate CECT examination of the abdomen followed by repeated clinical examinations. In about 40% of patients with pancreatic injury, the initial CECT can be normal, although a sensitivity of 87% and specificity of 98% with the new generation helical CECT has been reported.[3,13] Phelan et al[16] stated that even multi-detector CECT scan can fail to identify pancreatic injury accurately. When it detects pancreatic injury, it cannot grade it accurately for the presence or absence of pancreatic duct injury. ERCP has been until recently the most accurate method for detecting pancreatic duct trauma in the physiologically stable patient, by demonstrating extravasations of contrast medium from the pancreatic duct system.[4,17] Patients with hyper-amylasaemia, persistent abdominal pain and questionable abdominal CECT findings, who are being considered for non-operative management, should have the integrity of the duct system demonstrated by ERCP. However, if ERCP is to be carried out in lieu of operative exploration, it must be performed urgently within 12–24 hrs of injury, as further delays will jeopardize subsequent care. ERCP may be of value in cases with delayed presentation or injuries missed by CECT scan. It is also valuable in defining the nature and extent of damage to the duct, and in planning appropriate surgical correction (open surgery, internal trans-pancreatic duct stenting, trans-ductal drainage) for those patients who develop post-injury complications, such as pseudocysts or distal chronic pancreatitis.[3] Bradley et al[12] demonstrated a correlation between grades of pancreatic injury and outcome using the AAST-OIS system.[18] They concluded that ductal status is an important predictor of outcome in pancreatic trauma and is essential for establishing the basis for treatment decision. A delay in diagnosis of pancreatic injury has been demonstrated to increase pancreas related morbidity and mortality.[19,20] Operative management of patients with pancreatic injury has been described as dependent on main pancreatic duct status demonstrated by ERCP or magnetic retrograde cholangiopancreatogram (MRCP).[20-22] Management strategies for pancreatic trauma are very clear in surgical literature. Surgical intervention is always indicated for pancreatic blunt injury with use of wide drainage of the pancreas for grades I and II, low grade blunt pancreatic injury (LGBPI) and complex surgical intervention for grades III, IV, and V, high grade blunt pancreatic injury (HGBPI).2 With the evolution and general acceptance of non-operative management for solid organ injury, the management of blunt pancreatic injury which consists of surgical intervention even for the hemodynamically stable (HDS) patients with low grade pancreatic injuries needs to be further explored. There should not be any question, that once a high grade pancreatic injury is diagnosed on CECT scan, ERCP or MRCP, definitive surgical intervention is mandatory, since delay in diagnosis and treatment is associated with an increase in morbidity and mortality.[1] Duchesne et al[23] stated that non-operative management of LGBPI diagnosed by CECT scan was successful in the majority of hemodynamically stable (HDS) patients, with low morbidity and mortality. Hence they proposed that ERCP or MRCP should be done to rule out pancreatic duct injury in this group of patients. Patients with pancreatic duct injury on ERCP or MRCP and HGBPI should be dealt with surgery. ERCP and stent therapy may avoid surgery in the acute stage, and may be used as another choice for acute grade IV pancreatic injury. However the role of pancreatic duct stent is uncertain for acute grade III pancreatic injury due to variant outcome and long term duct strictures.[24] Complications are common in patients of pancreatic trauma and after operative treatment, ranging from 26 to 86%.[4,6,9,24,25] However higher AAST-OIS scores do not always translate into higher overall complications.25 Intra-abdominal abscess formation is one of the most common complications after pancreatic trauma. The reported incidence ranges from 10 to 25%, depending on the number and type of associated injuries.[29]
HGBPI and presence of an associated injury to the bowel increases the incidence.[9,25,26] These abscesses are mostly treated with radiology guided drainage and rarely by reoperation. [5,11] Pancreatic fistula is a common complication after operative repair of a major pancreatic injury.[4,9] The reported incidence ranges from 5 to 37%.[4,9,24,26-29] Provided adequate external drainage and nutritional support have been established, most fistulae resolve spontaneously within 1 or 2 weeks after injury. Fistulae secondary to major disruption of the pancreatic duct can generally be sealed by endoscopic stenting. If this fails, a distal pancreatectomy is recommended for fistulae of the neck, body and tail, and a Roux-en-Y loop to the head of the pancreas for fistulae of the head. Pseudocyst formation resulting from pancreatic trauma can present weeks or months after the original injury. If there is a disruption of the duct, endoscopic drainage and stenting or internal surgical drainage may be considered and in some cases distal pancreatectomy may be required.[30,31] In our study one patient developed a pseudocyst, and it was managed by internal drainage (cystogastrostomy). Al Ahmadi[32] reported endocrine deficiency in 16% patients of post-pancreatic trauma and no case with exocrine deficiency was reported. Buchler et al[33] reported that the peri-operative administration of octreotide reduced typical post-operative complications after pancreatic resection, particularly in the presence of tumors. Amirata et al[34] reported that the prophylactic use of octreotide was associated with no pancreatic complications and no negative sequelae. We use octreotide only in the presence of an established pancreatic fistula and not prophylactically.
In conclusion, pancreatic trauma is rare and it is more frequent among young male individuals and more are commonly inflicted by motor vehicles accidents. Hemodynamically stable patients with LGBPI and selected cases of HGBPI can be managed successfully by non-operative management with ERCP and stenting. Most patients with HGBPI and those with penetrating injuries needs surgical intervention, which may vary from simple external drainage to pancreaticoduodenectomy depending on the location, grade of injury and condition of the patient.
References
- Kao LS, Bulger EM, Parks DL, Byrd GF, Jurkovich GJ. et al. Predictors of morbidity after traumatic pancreatic injury. J Trauma. 2003;55:898–905.
- Patton JH Jr, Lyden SP, Croce MA, Pritchard FE, Minard G, Kudsk KA, et al. Pancreatic trauma: a simplified management guideline. J Trauma. 1997;43:234–41.
- Jurkovich G, Bulger EM. Duodenum and pancreas. In: Mattox KL, Moore ME, Feleciano DV, editors. Trauma. New York: McGraw-Hill Companies; 2004. p. 709–34.
- Wind P, Tiret E, Cunningham C, Frileux P, Cugnenc PH, Parc R. Contribution of endoscopic retrograde pancreatography in management of complications following distal pancreatic trauma. Am Surg. 1999;65:777–83.
- Lin BC, Chen RJ, Fang JF, Hsu YP, Kao YC, Kao JL. Management of blunt major pancreatic injury. J Trauma. 2004;56:774–8.
- Wolf A, Bernhardt J, Patrzyk M, Heidecke CD. The value of endoscopic diagnosis and the treatment of pancreas injuries following blunt abdominal trauma. Surg Endosc. 2005;19:665–9.
- Takishima T, Sugimoto K, Hirata M, Asari Y, Ohwada T, Kakita A. Serum amylase level on admission in the diagnosis of blunt injury to the pancreas; its significance and limitations. Ann Surg. 1997;226:70–6.
- Boffard KD, Brooks AJ. Pancreatic trauma—injuries to the pancreas and pancreatic duct. Eur J Surg. 2000;166:4–12.
- Vasquez JC, Coimbra R, Hoyt DB, Fortlage D. Management of penetrating pancreatic trauma: an 11-year experience of a level- 1 trauma center. Injury. 2001;32:753–9.
- Asensio JA, Petrone P, Roldán G, Kuncir E, Demetriades D. Pancreaticoduodenectomy. a rare procedure for the management of complex pancreaticoduodenal injuries. J Am Coll Surg. 2003;197:937–42.
- Feliciano DV. Abdominal trauma. In : Schwartz SI, Ellis H, editors. Maingot’s abdominal operations. 9th edition. East Norwalk: Appleton & Lange; 1989. p. 457–512.
- Bradley EL 3rd, Young P, Chang MC, Allen JE, Baker CC, Meredith W, et al. Diagnosis and initial management of blunt pancreatic trauma: guidelines from a multiinstitutional review. Ann Surg. 1998;227:861–9.
- Fleming WR, Collier NA, Banting SW. Pancreatic trauma: Universities of Melbourne HPB Group. Aust N Z J Surg. 1999;69:357–62.
- Wisner D, Wold R, Frey C. Diagnosis and treatment of pancreatic injuries. An analysis of management principles. Arch Surg. 1990;125:1109–13.
- Liu KJ, Atten MJ, Lichtor T, Cho MJ, Hawkins D, Panizales E, et al. Serum amylase and lipase elevation is associated with intracranial events. Am Surg. 2001;67:215–9; discussion 219–20.
- Phelan HA, Velmahos GC, Jurkovich GJ, Friese RS, Minei JP, Menaker JA, et al. An evaluation of multidetector computed tomography in detecting pancreatic injury: results of a multicenter AAST Study. J Trauma. 2009;66:641–6; discussion 646–7.
- Kim HS, Lee DK, Kim IW, Baik SK, Kwon SO, Park JW, et al. The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001;54:49–55.
- Moore EE, Cogbill TH, Malangoni MA, Jurkovich GJ, Champion HR, Gennarelli TA, et al. Organ injury scaling, II. Pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30:1427–9.
- Leppaniemi A, Haapiainen R, Kiviluoto T, Lempinen M. Pancreatic trauma: acute and late manifestations. Br J Surg. 1988;75:165–7.
- Hayward SR, Lucas CE, Sugawa C, Ledgerwood AM. Emergent endoscopic retrograde cholangiopancreatography. A highly specific test for acute pancreatic trauma. Arch Surg. 1989;124:745–6.
- Jurkovich GJ, Carrico CJ. Pancreatic trauma. Surg Clin North Am. 1990;70:575–93.
- Chandler C, Waxman K. Demonstration of pancreatic ductal integrity by endoscopic retrograde pancreatography allows conservative surgical management. J Trauma. 1996;40:466–8.
- Duchesne JC, Schmieg R, Islam S, Olivier J, McSwain N. Selective nonoperative management of low-grade blunt pancreatic injury: are we there yet? J Trauma. 2008;65:49–53.
- Lin BC, Liu NJ, Fang JF, Kao YC. Long-term results of endoscopic stent in the management of blunt major pancreatic duct injury. Surg Endosc. 2006;20:1551–5.
- Rickard MJ, Brohi K, Bautz PC. Pancreatic and duodenal injuries: keep it simple. ANZ J Surg. 2005;75:581–6.
- Tyburski JG, Dente CJ, Wilson RF, Shanti C, Steffes CP, Carlin A. Infectious complications following duodenal and/or pancreatic trauma. Am Surg. 2001;67:227–30; discussion 231.
- Buccimazza I, Thomson SR, Anderson F, Naidoo NM, Clarke DL. Isolated main pancreatic duct injuries spectrum and management. Am J Surg. 2006;191:448–52.
- Lopez PP, Benjamin R, Cockburn M, Amortegui JD, Schulman CI, Soffer D, et al. Recent trends in the management of combined pancreatoduodenal injuries. Am Surg. 2005;71:847–52.
- Jurkovich GJ, Duodenum and pancreas. In: Mattox KL, Feliciano DV, Moore EE, editors, Trauma. East Norwalk; Appleton & Lange; 2005. p. 709–34.
- Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Endoscopic management of pancreatic pseudocysts. Br J Surg. 1997;84:1638–45.
- Beckingham IJ, Krige JE, Bornman PC, Terblanche J. Long term outcome of endoscopic drainage of pancreatic pseudocysts. Am J Gastroenterol. 1999;94:71–4.
- Al-Ahmadi K, Ahmed N. Outcomes after pancreatic trauma: experience at a single institution. Can J Surg. 2008;51:118–24.
- Büchler M, Friess H, Klempa I, Hermanek P, Sulkowski U, Becker H, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163:125–30; discussion130–1.
- Amirata E, Livingston DH, Elcavage J. Octreotide acetate decreases pancreatic complications after pancreatic trauma. Am J Surg. 1994;168:345–7.
|