Vimal K Paliwal1, Pankaj K Gupta1, Praveer Rai2, Ritu Verma3
Departments of Neurology,1 Gastroenterology,2 and Pathology,3
Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Lucknow – 226014, Uttar Pradesh, India
Corresponding Author:
Dr. Vimal Kumar Paliwal
Email: dr_vimalkpaliwal@rediffmail.com
48uep6bbphidvals|519 48uep6bbph|2000F98CTab_Articles|Fulltext Systemic fungal infections are a less recognized complication of decompensated cirrhosis.[1] Recurrent behavioural abnormalities in cirrhotics are considered as episodes of hepatic encephalopathy. Meningitis is rarely suspected in these patients especially with lack of meningeal signs.[2] We present a patient with hepatitis C related decompensated cirrhosis of liver who developed recurrent altered mental status secondary to cryptococcal meningitis. We also discuss the possible immunological circumstances related to hepatic failure that may predispose to severe cryptococcal infection.
Case report
A 48-year-old lady presented with altered sensorium after an episode of generalized tonic-clonic seizures. There was no preceding headache, vomiting, fever or previous seizures. She had been diagnosed 2 years ago with hepatitis C virus related cirrhosis of liver with portal hypertension and grade 3 esophageal varices. On examination, she was in altered mental status (Glasgow coma scale (GCS) 9/15) (E2M4V3). Oral candidiasis was noticed. Neck rigidity was absent. On per abdominal examination, liver span was 10 cm. Spleen was just palpable below the left costal margin. Moderate ascites was noticed. Rest of the systemic examination was unremarkable. Her investigations revealed a haemoglobin of 8.5 gm/dl, TLC 3500 per mm3, neutrophils 60%, lymphocyte 40%, normal platelets, ESR 30 mm first hour (Westergren method) and microcytic hypochromic general blood picture. Renal and liver function tests, prothrombin time, serum ammonia and thyroid functions were normal. Serum proteins/albumin was 5.9/2.3 gm/dl. ELISA for HIV was negative. CD4+ count was 750/mm3. Thyroperoxidase antibodies were negative. Cerebrospinal fluid (CSF) examination revealed protein 55 mg/dl, cells 210 /mm3 and sugar 65 mg/ dl. CSF light microscopy with Gram, Ziehl Neelsen and India ink staining were negative. CSF culture for mycobacteria and fungi were sterile. CSF PCR for Mycobacterium tuberculosis, Varicella zoster, Herpes simplex type 1 and 2, and West Nile virus were negative. Blood and CSF ELISA for Japanese encephalitis and cryptococcal antigen were negative. MRI brain and electroencephalography was normal. By 14th day, she became afebrile and showed improvement in consciousness on treatment with broad spectrum antibiotics, fluconazole (for oral thrush) and supportive care and a repeat CSF examination revealed 20 cells (lymphocytes), protein of 140 mg%, and normal sugar. A possibility of nonspecific viral encephalitis was considered and the patient was discharged.
One month later, the patient returned with altered mental state again. There was no history of preceding headache, fever, behavioural changes or seizures. On examination, she had a GCS score of 9/15 (E3M4V2). Focal neurological signs and neck rigidity were absent. Systemic examination was unremarkable. Blood counts and biochemical examination was within normal limits. CSF revealed protein 133 mg/dl, sugar 56 mg/dl, cells 50/cumm (all lymphocytes), positive cryptococcal antigen and numerous cryptococcal cells on India ink preparation. Cryptococcus neoformans was recovered on CSF fungal culture. CSF bacterial and BACTEC culture were sterile. Ascitic fluid (transudative) was sterile on cultures. MRI brain showed dilated Virchow Robbin spaces in bilateral basal ganglia and numerous cryptococcomas in the central semiovale (Figures 1 & 2). The patient was instituted on conventional amphotericin B at 1 mg/kg body weight and 5-flucytosine at 2 gm/day. After one week, she developed spontaneous left hydropneumothorax which was drained by intercostal drainage. The pleural fluid was exudative but bacterial, BACTEC and fungal cultures were sterile. Anti-tubercular treatment was added on an empirical basis. The chest tube was removed after 2 weeks with no recurrence of hydropneumothorax. Despite improvement in CSF cell counts, the patient remained stuporous. Repeat CSF fungal cultures at 2 weeks showed heavy growth of Cryptococcus neoformans. Repeat MRI did not reveal hydrocephalus or infarcts. Amphotericin B and 5- flucytosine was continued along with the four drug anti-tubercular treatment. On 30th day of her illness, the patient developed sudden cardiac arrest and could not be revived.
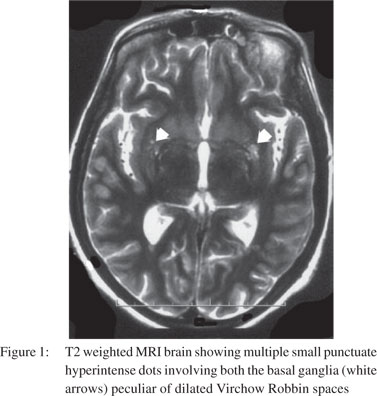
Discussion
Cirrhosis is a common yet under-recognized predisposing condition for cryptococcal meningitis.[3-7] The precise mechanisms that predispose cirrhotics to develop cryptococcal meningitis are not known. Cryptococcus spp. invokes innate immunity by interaction with toll like receptors on host cells and through cryptococcal mannoproteins, it stimulates cell mediated immunity. Activated CD4+, CD8+ T cells and Th1 cytokines prevent cryptococcal dissemination.[8] In decompensated cirrhosis, monocyte derived proinflammatory cytokines like interleukin 1â, interleukin 12, tumor necrosis factor á and HLA-DR expression on monocytes is markedly reduced and produces a state of “immune paralysis” as seen in late stage of sepsis that may predispose to cryptococcal dissemination.[9,10]
Once disseminated, Cryptococcus spp. have a predilection for the brain owing to their requirement of L-dopa which is needed as a substrate for producing melanin. The latter protects the fungal cells from oxidative killing. With cell mediated immunity compromised, the brain gets severely infected and with impaired opsonisation by inflammatory cells, more organisms remain extracellular then intracellular.[9] Both these phenomena of immunological breakdown are evident in our patient who developed multiple cerebral cryptococcomas, and owing to a very high CSF fungal load, showed heavy growth of Cryptococcus neoformans on repeat CSF cultures. The cryptococcal infection could not be isolated from CSF during her first hospital admission. This suggests that even crytpococcal inocula small enough to miss detection by current techniques may produce meningoencephalitis in immunocompromised states.
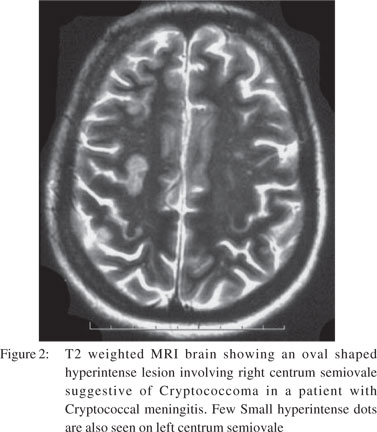 Other causes of recurrent encephalopathy not found in our patient were hepatic encephalopathy, electrolyte imbalance, Hashimoto’s encephalitis, limbic encephalitis, Mollaret’s meningitis, non-convulsive status epilepticus and septic encephalopathy. Recurrent encephalopathy in cryptococcal meningitis may occur due to inadequate treatment during consolidation phase as in our case. Spontaneous, transient improvement seen during the initial presentation was possibly due to milder disease, fluconazole therapy and improvement in general condition of our patient.
Hepatitis C and systemic cryptococcal co-infections have been reported previously.[10-13] Both organisms share certain pathophysiological characteristics like long incubation period, both down regulate organism specific immunity to persist in human tissue for years.[14-17] However, whether hepatitis C virus promotes cryptococcal persistence/dissemination in human tissue is not known.
References
Other causes of recurrent encephalopathy not found in our patient were hepatic encephalopathy, electrolyte imbalance, Hashimoto’s encephalitis, limbic encephalitis, Mollaret’s meningitis, non-convulsive status epilepticus and septic encephalopathy. Recurrent encephalopathy in cryptococcal meningitis may occur due to inadequate treatment during consolidation phase as in our case. Spontaneous, transient improvement seen during the initial presentation was possibly due to milder disease, fluconazole therapy and improvement in general condition of our patient.
Hepatitis C and systemic cryptococcal co-infections have been reported previously.[10-13] Both organisms share certain pathophysiological characteristics like long incubation period, both down regulate organism specific immunity to persist in human tissue for years.[14-17] However, whether hepatitis C virus promotes cryptococcal persistence/dissemination in human tissue is not known.
References
- Razafimahefa SH, Rabenjanahary TH, Rakotoarivelo RA, Ramilitiana B, Ramanampamonjy RM, Rajaona HR. Causes of death in a sample of cirrhotic patients from Madagascar. Med Trop (Mars). 2010;70:163–5.
- Planas R, Ballesté B, Alvarez MA, Rivera M, Montoliu S, Galeras JA, et al. Natural history of decompensated hepatitis C virusrelated cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–30..
- França AV, Carneiro M, dal Sasso K, Souza Cda S, Martinelli A. Cryptococcosis in cirrhotic patients. Mycoses. 2005;48:68–72.
- Pasqualotto AC, Bittencourt Severo C, de Mattos Oliveira F, Severo LC. Cryptococcemia. An analysis of 28 cases with emphasis on the clinical outcome and its etiologic agent. Rev Iberoam Micol. 2004;21:143–6.
- Chuang YM, Ku SC, Liaw SJ, Wu SC, Ho YC, Yu CJ, et al. Disseminated Cryptococcus neoformans var. grubii infections in intensive care units. Epidemiol Infect. 2010;138:1036–43.
- Yehia BR, Eberlein M, Sisson SD, Hager DN. Disseminated cryptococcosis with meningitis, peritonitis, and cryptococcemia in a HIV-negative patient with cirrhosis: a case report. Cases J. 2009;2:170.
- Artru P, Schleinitz N, Gauzere BA, Artru S, Paganin F, Roblin X. Neuromeningeal cryptococcosis and alcoholic cirrhosis. Gastroenterol Clin Biol. 1997;21:78–81.
- Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2005;72:99–118.
- Xing T, Li L, Cao H, Huang J. Altered immune function of monocytes in different stages of patients with acute on chronic liver failure. Clin Exp Immunol. 2007;147:184–8.
- El-Serag HB, Anand B, Richardson P, Rabeneck L. Association between hepatitis C infection and other infectious diseases: a case for targeted screening? Am J Gastroenterol. 2003;98:167–74.
- Saif MW, Raj M. Cryptococcal peritonitis complicating hepatic failure: case report and review of the literature. J Appl Res. 2006;6:43–50.
- Flagg SD, Chang YJ, Masuell CP, Natarajan S, Hermann G, Mendelson MH. Myositis resulting from disseminated cryptococcosis in a patient with hepatitis C cirrhosis. Clin Infect Dis. 2001;32:1104–7.
- Sort P, Morales M, Gómez J, Parés A, Rodés J. Pleural empyema caused by Cryptococcus neoformans in a patient with liver cirrhosis. Gastroenterol Hepatol. 1996;19:302–4.
- Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond). 2007;112:141–55.
- Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, et al. Immune evasion by hepatitis C virus NS3/4A proteasemediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102:2992–7.
- Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. 2003;33:1957–67.
- Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–48.
|