Madhavan M, Vimalraj V, Selvakumar E, Jyothibasu D, Rosy Vennila, Jeswanth S, Ravichandran P, Surendran R
Institute of Surgical Gastroenterology and Liver Transplantation,
Government Stanley Medical College Hospital,
The Tamilnadu Dr. MGR Medical University,
Chennai, Tamilnadu, India
Corresponding Author:
Dr. V Vimalraj
Email: drvimmi@gmail.com
Abstract
Objective and background data: Reduction in cellular elements of blood, secondary to hypersplenism is an established component of non-cirrhotic portal hypertension. Prior transfusion of blood or blood components is frequently required for safe surgical intervention. Due to thrombocytopenia, epidural catheter insertion for effective and durable analgesia is not possible. The aim of the present study was to objectively demonstrate the gain in blood components following early ligation of splenic artery for splenectomy in shunt surgery. Methods: From Jan 2008 to July 2010, 30 patients underwent elective proximal spleno renal shunt for portal hypertension, for various indications and were analyzed prospectively. We followed the standard protocol of ligating the splenic artery in situ, first in the lesser sac. Proximal spleno shunt was done . After the surgical procedure and before extubation, an epidural catheter was placed for effective and durable analgesia. 5ml of venous blood was drawn in the following order of sequence: prior to induction of anesthesia, immediately after the ligation of splenic artery, 30 minutes after ligation of splenic artery and 30 minutes after splenectomy. Samples were sent for complete hemogram and values were analyzed in respective order. Patients requiring transfusion of blood or blood components during surgery were excluded from the study.
Results: 30 patients (M - 9, F- 21) with mean age of 29.4 years ( 11-60 years) were analyzed (NCPF- 20, EHPVO- 9, cirrhosis- 1). We objectively demonstrated a significant gain in RBCs (p=0.016) and platelets (p=0.000) using this standard protocol. As there were no intrinsic abnormalities in RBCs, red blood cell indices (MCV, MCH, MCHC) showed no changes as expected (p-0.9).
Conclusion: By following this standard protocol, in addition to reduction in blood loss there was a significant gain in RBCs and platelets. This gain allows the surgeon to perform the surgical procedure safely and the anesthetist to secure an epidural catheter immediately after surgery for effective and durable analgesia without prior transfusion.
|
48uep6bbphidvals|493 48uep6bbph|2000F98CTab_Articles|Fulltext Portal hypertension is one of the most common cause of upper gastrointestinal bleeding in both pediatric and adult population.[1-4] Shunt surgery (proximal spleno renal shunt) is still performed for portal hypertension in both children and dults at many centers with good results.[5-7] Hypersplenism is an established component of non cirrhotic portal hypertension.[8]
In congestive splenomegaly, the volume of the spleen may be ten times greater than the normal and 30-90% of platelets and 40% of erythrocytes may be located in the spleen.[9-10] Anemia and thrombocytopenia as a result of hypersplenism, frequently calls for transfusion prior to safe surgical intervention and epidural catheter insertion for effective and durable analgesia (centroneuraxial anesthesia is safe with platelets counts higher than 1,00,000 cells/cumm).[11] The aim of the present study was to objectively demonstrate the enlarged spleen’s ability to rapidly decongest via the splenic vein into the systemic circulation after splenic artery ligation during splenectomy and thereby causing significant gain in blood components.
Methods
A total of 328 patients of portal hypertension were managed at our institution from January 2008 to July 2010. Among them, 31 patients underwent elective proximal spleno renal shunt (PSRS) surgery for various indications (Table 1). One patient was excluded from the study due to excessive blood loss during surgery, which required transfusion. Remaining 30 patients were thus included in this study. Ethical committee approval was obtained for this study.
Portal hypertension in these 30 patients was due to NCPF in 20, EHPVO in 9 and cirrhosis with Child A status in 1 patient. Massive splenomegaly was present in 26 and moderate splenomegaly in 4 patients. Symptomatic hypersplenism was considered in patients having hypersplenism with evidence of bleeding manifestations such as gum bleeding, epistaxis, menorrhagia or ecchymosis. Among the patients with symptomatic hypersplenism, gum bleeding was noted in 5 (all were NCPF patients), epistaxis in two (NCPF – 1, EHPVO – 1) and menorrhagia in one (EHPVO group) patient.
 Exclusion criteria
Patients requiring blood or blood components during surgical procedure were excluded from this study.
Surgical methods
We followed the standard protocol of ligating the splenic artery in situ, first in the lesser sac, then proceeding with standard steps and finally ligating the splenic vein to complete the splenectomy. During this period the massive spleen was allowed to decongest via the splenic vein into the systemic circulation. Just before ligating the splenic vein to complete the splenectomy, manual compression of the enlarged spleen for a period of 3-5 minutes was done to augment the decongestion. Thereafter the shunt was performed using the standard protocol. After the surgical procedure and before extubation, an epidural catheter was placed for effective and durable analgesia.
Five ml of venous blood was drawn in the following order of sequence: 1) prior to induction of anesthesia, 2) immediately after ligation of the splenic artery, 3) 30 minutes after splenic artery ligation, and 4) 30 minutes after splenectomy. Samples were sent for complete hemogram and values were analyzed in the respective order of sequence. Hematocrit values were not included because of intravenous fluid administration during the surgical procedure.
Statistical analysis
Statistical analysis was done using SPSS v11.5 statistical package. Data were presented as mean (+ SD) / median (range) or number (percentage) as appropriate. Hematological changes prior and after splenic artery ligation were assessed using paired t test. A p value < 0.05 was considered statistically significant.
Exclusion criteria
Patients requiring blood or blood components during surgical procedure were excluded from this study.
Surgical methods
We followed the standard protocol of ligating the splenic artery in situ, first in the lesser sac, then proceeding with standard steps and finally ligating the splenic vein to complete the splenectomy. During this period the massive spleen was allowed to decongest via the splenic vein into the systemic circulation. Just before ligating the splenic vein to complete the splenectomy, manual compression of the enlarged spleen for a period of 3-5 minutes was done to augment the decongestion. Thereafter the shunt was performed using the standard protocol. After the surgical procedure and before extubation, an epidural catheter was placed for effective and durable analgesia.
Five ml of venous blood was drawn in the following order of sequence: 1) prior to induction of anesthesia, 2) immediately after ligation of the splenic artery, 3) 30 minutes after splenic artery ligation, and 4) 30 minutes after splenectomy. Samples were sent for complete hemogram and values were analyzed in the respective order of sequence. Hematocrit values were not included because of intravenous fluid administration during the surgical procedure.
Statistical analysis
Statistical analysis was done using SPSS v11.5 statistical package. Data were presented as mean (+ SD) / median (range) or number (percentage) as appropriate. Hematological changes prior and after splenic artery ligation were assessed using paired t test. A p value < 0.05 was considered statistically significant.
Results
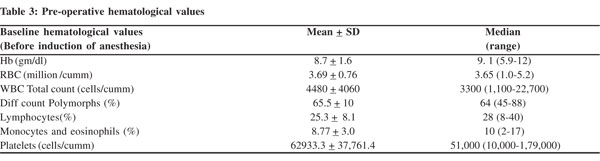
A total of 30 patients (9 males and 21 females) underwent elective proximal spleno renal shunt (PSRS) surgery for portal hypertension were enrolled and analyzed in this prospective study. Massive splenomegaly was present in 26 patients and moderate splenomegaly in 4. Lowest baseline platelet count was 10,000/cumm and highest was 1,79,000/cumm (Table 2). Baseline hematological values (prior to induction of anesthesia) were obtained and are tabulated in Table 3.
Likewise, the lowest hemoglobin was 5.9 gm/dl while the highest was 12gm/dl; and the lowest RBC count was 1.9 million/ cumm with a highest of 5.17 million/cumm. All other values were analyzed in their respective order . After ligation of the splenic artery, we observed a rise in all hematological parameters except lymphocytes and eosinophils (Table 4). Of these parameters a statistically significant rise was noted only in RBC and platelet counts. This statistically significant rise in the RBC and platelet counts was consistently noted across each measurement made in sequence. Given the fact that there was no expected intrinsic abnormality in the RBCs, other hematological indices like MCV, MCH, MCHC did not show any changes (p=0.9). The overall gain in RBC counts from the time of ligation of splenic artery to 30 minutes after splenectomy was 1.00 ± 0.23 mill/cumm (mean ± SD), and the analogous rise in platelet counts was 1,09,500.0 ± 46765.9 cells/ cumm (mean + SD).
Discussion
Ligation of the splenic artery in the lesser sac is the first step during splenectomy to reduce the blood loss in proximal spleno renal shunt.[12,13] By performing this standard procedure, we successfully achieved a gain in blood components along with a significant reduction in blood loss. We also noted that this gain is rapid and commences immediately after splenic artery ligation. Although all components except lymphocytes and eosinophils showed a rise, only RBCs and platelets showed statistically significant increase in levels. The enlarged spleen’s ability to decongest via the splenic vein into the systemic circulation was the reason for this gain in blood components. Since our study cohort had only one patient with cirrhosis, a comparison between cirrhotic versus non-cirrhotic portal hypertension was not possible. Although the RBC counts showed a significant gain, the hemoglobin rise was not consistent across the sequence of tests. This was probably due to administration of intravenous fluids during the time of surgery, and hence hematocrit values were not included in our study.
An earlier study by Gazula et al[14] also demonstrated a similar gain in hemoglobin, hematocrit, platelets and RBCs following early ligation of splenic artery during splenectomy in pediatric patients with EHPVO. In their study, the investigators waited for a period of 30 minutes to allow the spleen to decongest and no intravenous fluids were administered during this period. But in our study, we ligated the splenic artery first and then proceeded with the subsequent standard steps without a waiting period. Before ligating the splenic vein to complete the splenectomy, manually compression of the spleen was performed to augment decongestion and this was observed to aid in the rise of counts after splenectomy. Interestingly, we observed the raise in RBC and platelet counts immediately after the splenic artery ligation, probably due to rapid decongesting capacity of the enlarged spleen and autotransfusion of the cellular elements into the patient’s systemic circulation. In the latter part of our study we undertook shunt surgery for a patient with a platelet count of 10,000/cumm. Immediately after splenic artery ligation the patient’s platelet count rose to 35,000/cumm. No bleeding episode was encountered during surgery and an epidural catheter was also placed safely. Post-operative outcome was also good for this patient.

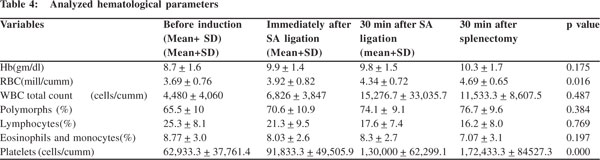 The net gain in RBC counts from ligation of splenic artery to 30 minutes after splenectomy was 1.00 ± 0.23 mill/cumm (mean + SD), and was 1,09,500.0 ± 46765.9 cells/cumm (mean + SD) for platelets. Platelet concentrate produced from a unit of whole blood contains on average, 7500 cells/cumm and has been shown to increase the platelet count by 5,000-10,000 cells/ cumm in a 70 kg recipient.[15] The platelet gain in our study was equivalent to transfusion of at least 8 units of platelets in a 70 kg adult. This gain enables the surgeon to perform surgery safely, even when the initial platelet count is low (significant bleeding usually does not occur with platelet counts greater than 50,000 cells/cumm);[16] and allows the anesthetist to insert an epidural catheter after surgery for effective and durable analgesia, without any need for prior transfusion (centroneuraxial anesthesia is safe at platelet counts higher than 1,00,000 cells/cumm).[11] In addition to conservation of blood components, this procedure also helps circumvent the hazards of multiple transfusions.[17]
The relationship between the volume of spleen and the risein counts was not analyzed in our study, because most of our patients presented with massive splenomegaly. But according to a previous study, there appears to be a positive correlation between these two variables.[14] Interestingly, instead of surgically ligating the splenic artery, the efficacy of radiologically guided embolization of the splenic artery can also be investigated to study pre-operative increase in platelet counts. Whether this ligation can be used to treat other thrombocytopenic conditions such as idiopathic thrombocytopenic purpura can also be analyzed in the future. Our study included only a small number of patients, and remains the limitation of our study. Further well powered studies are warranted to clarify this issue. By adopting this simple and feasible protocol, not only critical blood loss can be reduced but also a significant gain in RBCs and platelets can be achieved in these patients. This hematological buffer allows the surgeon to perform the surgical procedure safely and the anesthetist to carry out epidural catheter insertion for post-operative analgesia without any additional need for transfusion. Thus this elegant procedure not only conserves the patient’s blood components but also prevents transfusion associated hazards.
References
The net gain in RBC counts from ligation of splenic artery to 30 minutes after splenectomy was 1.00 ± 0.23 mill/cumm (mean + SD), and was 1,09,500.0 ± 46765.9 cells/cumm (mean + SD) for platelets. Platelet concentrate produced from a unit of whole blood contains on average, 7500 cells/cumm and has been shown to increase the platelet count by 5,000-10,000 cells/ cumm in a 70 kg recipient.[15] The platelet gain in our study was equivalent to transfusion of at least 8 units of platelets in a 70 kg adult. This gain enables the surgeon to perform surgery safely, even when the initial platelet count is low (significant bleeding usually does not occur with platelet counts greater than 50,000 cells/cumm);[16] and allows the anesthetist to insert an epidural catheter after surgery for effective and durable analgesia, without any need for prior transfusion (centroneuraxial anesthesia is safe at platelet counts higher than 1,00,000 cells/cumm).[11] In addition to conservation of blood components, this procedure also helps circumvent the hazards of multiple transfusions.[17]
The relationship between the volume of spleen and the risein counts was not analyzed in our study, because most of our patients presented with massive splenomegaly. But according to a previous study, there appears to be a positive correlation between these two variables.[14] Interestingly, instead of surgically ligating the splenic artery, the efficacy of radiologically guided embolization of the splenic artery can also be investigated to study pre-operative increase in platelet counts. Whether this ligation can be used to treat other thrombocytopenic conditions such as idiopathic thrombocytopenic purpura can also be analyzed in the future. Our study included only a small number of patients, and remains the limitation of our study. Further well powered studies are warranted to clarify this issue. By adopting this simple and feasible protocol, not only critical blood loss can be reduced but also a significant gain in RBCs and platelets can be achieved in these patients. This hematological buffer allows the surgeon to perform the surgical procedure safely and the anesthetist to carry out epidural catheter insertion for post-operative analgesia without any additional need for transfusion. Thus this elegant procedure not only conserves the patient’s blood components but also prevents transfusion associated hazards.
References
- Cox K, Ament ME. Upper gastrointestinal bleeding in children and adolescents. Pediatrics. 1979;63:408–13.
- Yachha SK, Khanduri A, Sharma BC, Kumar M. Gastrointestinal bleeding in children. J Gastroenterol Hepatol. 1996;11:903–7.
- Anand CS, Tandon BN, Nundy S. The causes, management and outcome of upper gastrointestinal haemorrhage in an Indian hospital. Br J Surg. 1983;70:209–11.
- Venbrux AC. Upper Gastrointestinal bleeding; Diagnostic evaluation and management. In: SCVIR Syllabus, Thoracic and Visceral Vascular Interventions. Lippincott Williams and Wilkins; 1996. p. 235–46.
- Prasad AS, Gupta S, Kohli V, Pande GK, Sahni P, Nundy S. Proximal splenorenal shunts for extrahepatic portal venous obstruction in children. Annals of Surgery. 1994;219:193–6.
- Rao KL, Goyal A, Menon P, Thapa BR, Narasimhan KL, Chowdhary SK, Extrahepatic portal hypertension in children: observations on three surgical procedures. Pediatr Surg Int. 2004;20:679–84.
- Orloff MJ, Orloff MS, Girard B, Orloff SL. Bleeding esophagogastric varices from extrahepatic portal hypertension: 40 years’ experiencewith portal-systemic shunt. J Am Coll Surg. 2002;194:717–28; discussion 728–30.
- Sarin SK, Kumar A. Noncirrhotic portal hypertension. Clin Liver Dis. 2006;10:627–51.
- Jesic R, Radevic B, Sagic D, Culafic D, Pavlovic A, Cvejic T. Treatment of hypersplenism in liver cirrhosis. Arch Gastroenterohepatol. 2002;21:3–4.
- Yongxiang W, Zongfang L, Guowei L, Zongzheng J, Xi C, Tao W. Effects of splenomegaly and splenic macrophage activity in Hypersplenism due to cirrhosis. Am J Med. 2002;113:428–31.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1506–11.
- Linton RR, Jones CM, Volwiler W. Portal hypertension; the treatment by splenectomy and splenorenal anastomosis with preservation of the kidney. Surg Clinics of North America. 1947;27:1162–70.
- Linton RR . Atlas of Vascular Surgery . Philadelphia: WB Saunders; 1973.p.168–91.
- Gazula S, Pawar DK, Seth T, Bal CS, Bhatnagar V. Extrahepatic portal venous obstruction: The effects of early ligation of splenic artery during splenectomy. J Indian Assoc Pediatr Surg. 2009;14:194–9.
- Consensus Conference. Platelet Transfusion Therapy. JAMA. 1987;257:1777–80.
- Joseph J, Wizorek, Brain G, Rubin, Morey A. Blinder. Hemostasis and transfusion therapy. In: Gerard M. Doherty, Jennifer K. Lowney, John E. Mason, Scott I. Reznik , Michael A. Smith, editors. The Washington Manual of Surgery. Lippincott Williams & Wilkins; 2002. p. 31–7.
- Williamson LM, Lowe S, Love EM, Cohen H, Soldan K, McClelland DB, et al. Serious hazards of transfusion (SHOT) initiative: analysis of the first two annual reports. BMJ. 1999;319:16–9.
- Lee CM, Leung TK, Lee WH, Shen LK, Liu JD, Chang CC, et al World J Gastroenterol. 2007;13:619–22.
|