Appasani S,< offshoresup>1 Kochhar S,2 Nagi B,1 Gupta V,3 Kochhar R1
Departments of Gastroenterology,1
General Surgery,3
Postgraduate Institute of Medical Education and Research;
Department of Radiodiagnosis,2
Government Medical College and Hospital,
Chandigarh, India
Corresponding Author:
Dr. Rakesh Kochhar
Email: dr_kochhar@hotmail.com
Abstract
Intrinsic or extrinsic obstruction of the pyloric channel or duodenum either by benign or malignant diseases leads to gastric outlet obstruction. With improvement in science and technology, the spectrum of gastric outlet obstruction has changed from peptic ulcer disease to corrosives and malignant diseases. Newer investigations like computerized tomography and endoscopy have supplemented the previous clinical tests like saline load test and barium series. Improvised treatment modalities like endoscopic balloon dilatation and endoscopic incision have circumvented the use of surgery which was the gold standard for management of gastric outlet obstruction. Newer modalities like biodegradable stents have an upcoming role in the management.
|
48uep6bbphidvals|461 48uep6bbph|2000F98CTab_Articles|Fulltext Gastric outlet obstruction (GOO) represents a clinical and pathophysiological consequence of any disease process which produces mechanical impediment to gastric emptying. Intrinsic or extrinsic obstruction of the pyloric channel or duodenum is the usual pathophysiology of GOO and the mechanism of obstruction depends upon the underlying etiology. Classification of diseases causing GOO into 2 well-defined groups of benign and malignant facilitates management and treatment. Historically GOO has been considered a disease process synonymous with chronic peptic ulcer disease. However since the advent of proton pump inhibitors, the complications from peptic ulcer disease have drastically decreased with a change in ratio between benign and malignant gastric outlet obstruction.[1] Most patients with GOO present with vomiting as their cardinal symptom and tend to develop dehydration and dyselectrolytemia if untreated. Malnutrition and weight loss are frequent when the condition approaches chronicity and are most significant in patients with malignant etiologies.
Epidemiology
The incidence of gastric outlet obstruction is not known precisely. Though malignancy remains a common cause of GOO in adults, a significant number of patients with GOO have benign causes.[2,3] Until the late 1970s benign disease was responsible for a majority of cases of GOO in adults, while malignancy accounted for only 10 to 39 percent of cases. By contrast, in the recent decades 50 to 80 percent cases have been attributed to malignancy.[4,5]
The incidence of GOO has been reported to be less than 5% in patients with peptic ulcer disease (PUD), which was earlier the leading benign cause of the problem. The incidence of GOO in patients with peripancreatic malignancy, the most common malignant etiology, has been reported between 15- 20%. In 1990, as many as 2000 operations for GOO were performed annually.[6] Updated estimates are not available, but the need for surgery is thought to have declined because of advancements in endoscopic methods to treat GOO (such as dilatation and stenting). Even in a developing country like India, malignancy is the commonest cause of gastric outlet obstruction. Misra et al reported that 75% of their patieents with GOO had a malignant cause.[7]
Etiology
Peptic ulcer disease and corrosive ingestion are the leading causes of benign gastric outlet obstruction.[8] Nonsteroidalantiinflammatory drugs (NSAID) and opium addiction are rare causes of gastric outlet obstruction.[9] Other benign causes are gastric polyps, pyloric stenosis, congenital duodenal webs, gallstone obstruction (Bouveret’s syndrome), pancreatic pseudocysts, and bezoars (Table 1).[8]
Peptic ulcer disease (PUD)
PUD once the most common cause of GOO, has witnessed significant decline in incidence due to the discovery of Helicobacter pylori and proton pump inhibitors (PPI).[10,11] Obstruction is now the least common complication of peptic ulcer disease, occurring in approximately 2 percent of cases. A comparison of data from previous Indian studies suggests a changing trend of peptic ulcer with respect to age and sex distribution, the ratio between DU and GU, and complications of peptic ulcer.[12] GOO occurs in both acute and chronic peptic ulcers. In acute stage inflammation induced edema, spasm, tissue deformation and pyloric dysmotility lead to GOO. In chronic PUD scarring and tissue remodeling lead to GOO. Gastric atony developing after prolonged obstruction contributes to gastric retention. While most cases are associated with duodenal/pyloric channel ulceration, gastric ulceration accounts for only 5% of cases.
 Corrosive injury
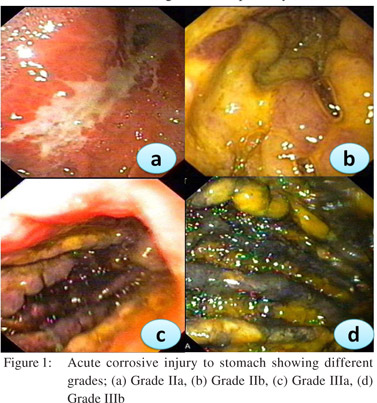
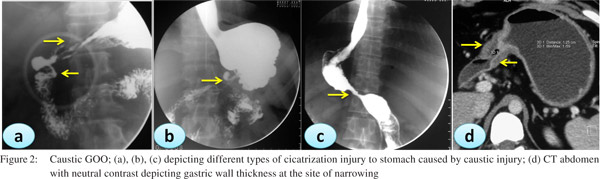
Caustic ingestion including both acid and alkali can lead to GOO due to antral or pyloric scarring and rarely due to duodenal scarring (Figures 1, 2). Chronic GOO manifests before or along with the esophageal obstruction. Associated esophageal injuries mask the presentation of GOO in caustic injury. The maximum damage occurs along the lesser curvature and the pre-pyloric area. Presence of food in the stomach determines the nature of injury in caustic injury. In fasting state most of the damage occurs to the pylorus and antrum while in the fed state the body gets commonly involved causing hour glass cicatrization. East-West differences do exist with caustic injury too. In India gastric involvement is seen more often due to acid ingestion whereas in the West esophageal involvement is more commonly due to lye ingestion.[13] The incidence of coexistent esophageal injury is variable from 20%[14,15] to 60%.[16] Isolated GOO in caustic ingestion is reportedly seen in about one third of thecases. Indian studies have reported an incidence of 44.4% in acid ingestion and 36.8% in alkali ingestion.[14,15]
Corrosive injury
Caustic ingestion including both acid and alkali can lead to GOO due to antral or pyloric scarring and rarely due to duodenal scarring (Figures 1, 2). Chronic GOO manifests before or along with the esophageal obstruction. Associated esophageal injuries mask the presentation of GOO in caustic injury. The maximum damage occurs along the lesser curvature and the pre-pyloric area. Presence of food in the stomach determines the nature of injury in caustic injury. In fasting state most of the damage occurs to the pylorus and antrum while in the fed state the body gets commonly involved causing hour glass cicatrization. East-West differences do exist with caustic injury too. In India gastric involvement is seen more often due to acid ingestion whereas in the West esophageal involvement is more commonly due to lye ingestion.[13] The incidence of coexistent esophageal injury is variable from 20%[14,15] to 60%.[16] Isolated GOO in caustic ingestion is reportedly seen in about one third of thecases. Indian studies have reported an incidence of 44.4% in acid ingestion and 36.8% in alkali ingestion.[14,15]
 Drugs
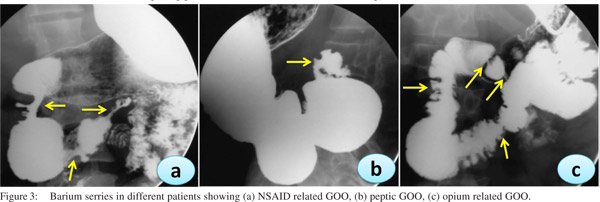
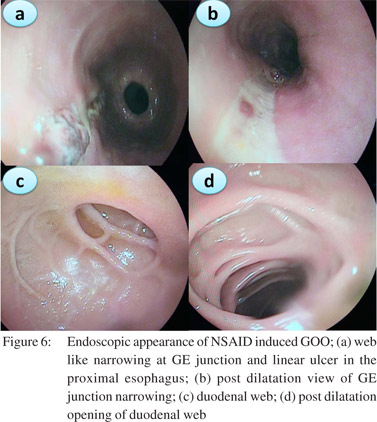
In the recent years we have noted an upsurge in NSAID and opium addiction cases presenting with GOO at our institution (Figure 3). Nonsteroidal anti-inflammatory drugs (NSAIDs) are known to be associated with various forms of gastrointestinal injuries including peptic ulcer disease, diaphragm disease of the bowel and protein losing enteropathy. We reported a series of 10 patients with NSAID related GOO.[9] The most common site of involvement was duodenum (5/10) followed by both pylorus and duodenum (4/10) and pylorus (1/10). Most of the strictures were short web-like and the mean (SD) number of strictures were 2.0 (0.94). Endoscopic balloon dilatation was successful in 90% (9/10) cases requiring mean (SD) 2.0 (1.6) sessions of dilatation to achieve target diameter of 15 mm and mean (SD) 5.3 (2.7) sessions to maintain it over a treatment period of 4.5 months (IQR 2-15 months) with no procedure-related complication or mortality. The predilection for duodenum rather than jejunum or ileum for these diaphragmlike strictures in our study could be due to the use of non enteric coated preparations. NSAIDs cause GOO by diminishing the levels of prostaglandin E2 causing pyloric edema and scarring and increasing histamine release leading to increased gastric secretion, reduction of mucosal absorption, and gastric motility disturbances.[17]
Inflammatory causes
Isolated gastroduodenal Crohn’s disease is uncommon, while majority have concomitant disease in the distal gastrointestinal tract. Clinically significant gastroduodenal Crohn’s occurs in fewer than 5% of patients. When present, about 60 percent of patients have continuous disease that involves the antrum, pylorus, and proximal duodenum.[18]
Gastroduodenal tuberculosis occurs in only 0.3 to 2.3 percent of patients with tuberculosis. GOO was the presenting feature in 61% of 23 patients with biopsy proven gastroduodenal TB.[19] The obstruction may be secondary to infiltration of the gastric antrum or duodenum or to extrinsic compression by adenopathy or a phlegmon. Chronic pancreatitis, groove pancreatitis, annular pancreas and to a lesser extent severe acute pancreatitis can cause GOO. Case series report an incidence of about 1-5%.[20,21] The obstruction develops due to spread of inflammatory exudates from the anterior pancreatic surface, which leads to inflammation and fibrosis in adjacent structures, most commonly the duodenum, jejunum, and transverse colon. Patients with duodenal obstruction often have associated pancreatic and biliary duct strictures.
Rare causes
Gastric bezoars, large gastric polyps, migration of PEG tube into the pyloric channel or duodenal bulb, gastric vovlulus, Bouveret’s syndrome due to impaction of gall stone in antrum and eosinophilic gastroenteritis are other rare causes of GOO.
Drugs
In the recent years we have noted an upsurge in NSAID and opium addiction cases presenting with GOO at our institution (Figure 3). Nonsteroidal anti-inflammatory drugs (NSAIDs) are known to be associated with various forms of gastrointestinal injuries including peptic ulcer disease, diaphragm disease of the bowel and protein losing enteropathy. We reported a series of 10 patients with NSAID related GOO.[9] The most common site of involvement was duodenum (5/10) followed by both pylorus and duodenum (4/10) and pylorus (1/10). Most of the strictures were short web-like and the mean (SD) number of strictures were 2.0 (0.94). Endoscopic balloon dilatation was successful in 90% (9/10) cases requiring mean (SD) 2.0 (1.6) sessions of dilatation to achieve target diameter of 15 mm and mean (SD) 5.3 (2.7) sessions to maintain it over a treatment period of 4.5 months (IQR 2-15 months) with no procedure-related complication or mortality. The predilection for duodenum rather than jejunum or ileum for these diaphragmlike strictures in our study could be due to the use of non enteric coated preparations. NSAIDs cause GOO by diminishing the levels of prostaglandin E2 causing pyloric edema and scarring and increasing histamine release leading to increased gastric secretion, reduction of mucosal absorption, and gastric motility disturbances.[17]
Inflammatory causes
Isolated gastroduodenal Crohn’s disease is uncommon, while majority have concomitant disease in the distal gastrointestinal tract. Clinically significant gastroduodenal Crohn’s occurs in fewer than 5% of patients. When present, about 60 percent of patients have continuous disease that involves the antrum, pylorus, and proximal duodenum.[18]
Gastroduodenal tuberculosis occurs in only 0.3 to 2.3 percent of patients with tuberculosis. GOO was the presenting feature in 61% of 23 patients with biopsy proven gastroduodenal TB.[19] The obstruction may be secondary to infiltration of the gastric antrum or duodenum or to extrinsic compression by adenopathy or a phlegmon. Chronic pancreatitis, groove pancreatitis, annular pancreas and to a lesser extent severe acute pancreatitis can cause GOO. Case series report an incidence of about 1-5%.[20,21] The obstruction develops due to spread of inflammatory exudates from the anterior pancreatic surface, which leads to inflammation and fibrosis in adjacent structures, most commonly the duodenum, jejunum, and transverse colon. Patients with duodenal obstruction often have associated pancreatic and biliary duct strictures.
Rare causes
Gastric bezoars, large gastric polyps, migration of PEG tube into the pyloric channel or duodenal bulb, gastric vovlulus, Bouveret’s syndrome due to impaction of gall stone in antrum and eosinophilic gastroenteritis are other rare causes of GOO.
Clinical manifestations
The most common clinical features of GOO include nausea and vomiting, epigastric pain, early satiety, abdominal distension and weight loss. The onset of symptoms varies depending upon the etiology of the obstruction. Symptoms generally occur abruptly with gallstone impaction, prolapse of a large gastric polyp, PEG tube migration and gastric volvulus. Other causes tend to follow a more indolent course. Patients with malignant disease may have a shorter duration of symptoms compared with those with benign disease. Benign causes of GOO most commonly presented with early satiety (53%) and bloating (50%) while malignant GOO presented more commonly with pain, vomiting and weight loss.[22]
Although commonly absent, the presence of recognizable food more than 8–12 hrs after eating is indicative of gastric retention. The average duration of symptoms is 1 month, although one-third of patients have symptoms for longer than 3 months. A subset of patients with chronic peptic ulcer disease has visceral hyposensitization and in these patients high grade outlet obstruction may be present without perceived gastric distress.
Features of weight loss and volume depletion should be looked for in a suspected case of GOO. Succussion splash, demonstrated by auscultation of a “splash” reflective of retained gastric material, if noted more than four hours after a meal is suggestive of GOO with a sensitivity of 50%.[23]
Investigations
Patients with recurrent vomiting may have electrolyte abnormalities including hypokalemia or a hypochloremicmetabolic alkalosis. Anemia and abnormal liver function tests may reflect the underrlying disease. Elevated serum gastrin levels due to distention-induced gastrin release (400 to 800 pg/ mL range) can occur and can lead to confusion with Zollinger- Ellison syndrome.
Plain radiographs may show an enlarged gastric air bubble which never crosses the midline since lesser curvature is a fixed structure. Small bowel may not show up due to paucity of air. Pancreatic calcification and/or calcified gall stone may be revealed. Contrast studies with water soluble contrast or barium can give clues to the etiology of the underlying disease. Failure of any contrast to pass into the small bowel suggests complete GOO. In view of risk of aspiration adequate decompression should be done before giving water soluble contrast. CT scan may reveal additional details, specially the pyloric or gastric wall thickness, lymph nodes or pancreatic lesion not visualized on routine imaging.
Endoscopy is often needed to establish the diagnosis and identify a specific cause along with a therapeutic benefit. Patients should fast for at least four hours before the procedure. Fasting for prolonged periods is not necessary and may increase the amount of retained fluid. Nasogastric tube suction or use of large bore Ewald tube, which can minimize the risk of aspiration, is recommended before endoscopy. Endoscopic biopsies often allow confirmation or exclusion of a malignant cause of GOO. There is an evolving role for the use of endoscopic ultrasound in the management of these disorders.
Saline load test
After gastric decompression, the saline load test[24] is helpful to evaluate mechanical outlet obstruction. The initial role of this test was to evaluate the need for surgery in patients with GOO. A 16 Fr nasogastric tube is placed in the stomach and the stomach contents are emptied with the in the right lateral position.750 mL of 0.9% NaCl is infused through the tube over 3–5 min and is aspirated after 30 minutes to measure the gastric residual volume. Presence of >400 mL is suggestive of definite gastric outlet obstruction, 300-400 mL - probable gastric outlet obstruction, 200–300 mL – indeterminate and less than 200 mL is adjudged normal.
Management
Symptomatic GOO needs hospitalization. Fluid resuscitation with normal saline and correction of dyselectrolytemia forms the major treatment in the initial stages. Metabolic alkalosis needs to be especially addressed along with hypokalemia. Nasogastric decompression should be initiated at admission. This would relieve the discomfort of distension, clear the field for endoscopic procedures and help in reduction of stomach capacity which is essential for surgery. Definitive management can be instituted after establishment of diagnosis and correction of underlying metabolic abnormalities. Fibrotic scarring in PUD and caustic injury can cause irreversible GOO which requires intervention. The only option in the past had been surgery which was performed in more than 75% of these patients. However with the advent of endoscopic balloon dilatation, major surgical interventions and associated morbidity can be avoided now.
Endoscopic balloon dilatation (EBD)
Benjamin et al[25,26] were the first to report the use of endoscopic balloon dilatation of the pylorus as a treatment for gastric outlet obstruction using a through-the-scope (TTS) 5 mm balloon with a 150 cm long catheter with good technical success and clinical outcome. Evidence proves that EBD is a safe and effective alternative in the management of GOO.[27,28,29,30,31,32,33,34,35,36,37,38] The initial TTS balloons were modified by radio opaque wire guidance to enhance their efficacy of negotiating difficult strictures. Single diameter and variable diameter balloons are available from 6 mm to 20 mm which are inflated with a hydrostatic device attached to a pressure gauge. The CRE and Quantum TTC ® balloon dilators have the advantage that the same balloon can be dilated to different diameters e.g. from 10 mm to 12 mm, or 15 mm to 18 mm, making the procedure simple. Single short strictures give the best results with balloon dilatation; therefore patients should be selected with appropriate imaging (barium studies / CT scan).
Patients should be prepared adequately for the procedure. A minimum of 4 hrs fasting with liquid diet for 24 hrs prior to the procedure would give adequate visualization of the pathology. In severe GOO lavage with wide bored tubes may be required to clear the endoscopic field. The guidewire is pushed out of the TTS balloon and is negotiated across the stricture such that the centre of stricture and balloon coincide with each other. Minimal air insufflations could be helpful in cicatrized stomachs with eccentric pylorus. During inflation care has to be taken that the balloon does not slip in or slip out of the stricture. Dilatation with different balloons or a single balloon with incremental diameters can be used in the same session. Monitoring of the dilatation could be done under fluoroscopy with a contrast mixed saline used for inflation of balloon. The balloon size used depends on the clinical scenario and endoscopist’s perception. Post-procedure the patient is monitored for signs and symptoms of perforation and bleeding for 4-6 h. In patients with suspected perforation a contrast study should be carried out immediately using water soluble contrast medium.The procedure is repeated 1-2 weekly until adequate dilatation of 15-18 mm is achieved (Figures 4,5,6).
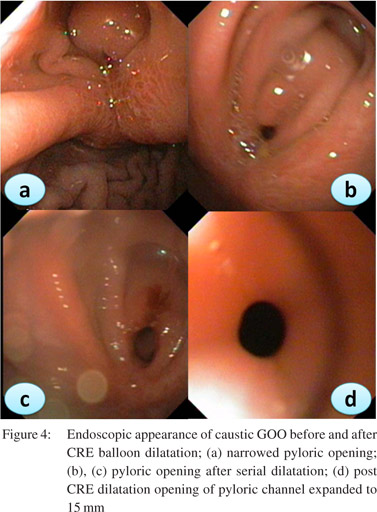
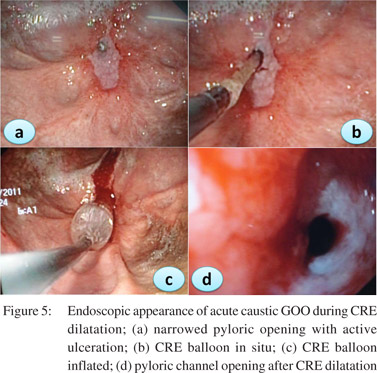
 Adequate nutritional support is mandatory for good clinical outcomes. A naso-jejunal tube feeding after acute corrosive injury (>Gr 11b endoscopically) is advocated in our practice. If successful dilatation can be carried out to 8-10 mm a homogenized liquid diet can be allowed orally ensuring adequate calorie and protein intake. Residue at each dilatation could be a marker of the adequacy of previous dilatations. In patients with peptic GOO maintenance of nutrition is not a big problem as response to EBD is prompt.
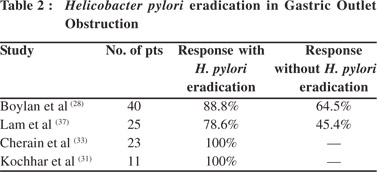
In one of our studies on benign GOO, we showed that patients with peptic GOO responded uniformly to EBD combined with eradication of H. pylori, and had good long term response as well over a mean follow-up period of 14.04 ± 9.79 months.[31] EBD in peptic GOO had variable results considering the confounding factors of H. pylori eradication, usage of PPI, NSAID abuse, etc. Immediate relief is noted with variable long term responses. Studies that looked for and eradicated H. pylori have reported a good long term response in 70-80% of patients over 9 to 98 months of follow up (Table 2).[28,31,33,37]In the absence of prospective RCTs, the available literature suggests that more than three-fourths of patients with peptic GOO respond to EBD and long term proton pump inhibitor usage may be required to prevent recurrences after H. pylori eradication. Caustic GOO is more difficult to dilate than peptic GOO. Such patients require more sessions and have more recurrences. Solt et al[29] have shown long term response in only one tthird of patients with caustic GOO. We, howwever, observed that out of 41 patients with caustic GOO, [39] patients responded to EBD with a mean (SD) 5.8 ± 2.6 dilations (range 2-13) to achieve the end point of 15 mm. The other 2 patients underwent surgery, one due to perforation and the other due to intractable pain.36 However the mean number of sessions were 2-13 in comparison to peptic GOO which required only 1-3 sessions.[31,36] We have recently shown that early EBD by an expert endoscopist could show better responses in acute caustic GOO.[39] Thirty patients underwent successful dilatations within 2 weeks (14.39 +/- 4.65 days) of acid ingestion to achieve the end point of 15 mm, requiring a median of 9 (range 3-18) dilatations over a period of 7 (range 1.5-16) weeks which persisted for a median 21 (range 3-72) months with no recurrence.
Other causes of GOO like tuberculosis, Crohn’s disease, and pancreatitis do respond to EBD. However they have more recurrences unless the basic disease is managed and adequately treated. EBD in these conditions had beensuccessful as reported by Misra et al[30] in tuberculosis and Kim et al[40] in Crohn’s disease. Anastomotic strictures following vertical band gastroplasty or gastric bypass surgery for morbid obesity have also been successfully dilated with balloon catheters endoscopically.[41]
Complications of EBD
Complications are uncommon with EBD.8 Bleeding and perforation are rarely reported for dilatation less than 15 mm. Increasing the balloon diameter beyond 15 mm is more likely to be associated with perforation. Self limiting pain is common with most EBD. In our study 19.5% of patients with caustic GOO had self-limiting pain during the procedure.[36]
Combination therapy
Intralesional steroids
Augmentation of EBD could be done with intralesional steroids injections and endoscopic incision. Intralesional steroid injections have been shown to inhibit stricture formation by interfering with collagen synthesis, fibrosis and chronic scarring.[42] Triamcinolone prevents the cross linking of collagen that results in scar contracture; so if the scar is stretched and steroid is injected into it, presumably the contracture will not occur.[43] Efficacy of steroids augmenting EBD in GOO has been demonstrated in two studies by Lee et al[44] and Kochhar et al.[45] We reported the use of steroids to augment dilatation in caustic GOO in 3 patients, one of whom was given steroids in the first dilatation itself.[45] All 3 patients had responded to dilatation in 1-2 sessions of injection.
Endoscopic incision
Endoscopic incision followed by EBD had been used and was successful in a few series of caustic GOO. Baron et al[46] used electrosurgical incision using a standard sphincterotomy and Hagiwara et al[47] used needle-knife radial incisions electrosurgically at gastroscopy combined with EBD. We have also used this technique in one of the patients with causticinduced GOO, who had become pregnant while on a dilation programme.[36]
Newer modalities
Placement of biodegradable stents in the enteral tract is an emerging alternative especially in caustic injury. A few case reports have shown efficacy of biodegradable stents in corrosive esophageal strictures.[48,49] Placement of self expanding metal stents (SEMS) is also an upcoming option for benign diseases. The probable role of SEMS is persistent dilatation for an extended period of time than what can be achieved with TTS balloons. However limited experience precludes the routine usage of such devices.
Surgery
Surgery forms the final option for patients presenting with refractory GOO. Most common surgeries for peptic strictures include vagotomy and antrectomy, vagotomy and pyloroplasty, truncal vagotomy and gastrojejunostomy, pyloroplasty and laparoscopic variants. Gastrojejunostomy (Billroth II reconstruction) can be considered in patients with preserved anatomy like peptic GOO, however with altered anatomy, Rouxen- Y jejunostomy would be the preferred option. Placement of a jejunostomy tube at the time of surgery should be considered, as patients with GOO are already malnourished. Also in chronically dilated conditions the stomach is slow to recover the normal rate of emptying. In peptic GOO gastrojejunostomy can be combined with truncal vagotomy and antrectomy. Laparoscopic gastrojejunostomy for the relief of GOO is associated with a smoother and more rapid postoperative recovery and shorter hospital stay compared with open surgery and should become the new gold standard.[50]
References
Adequate nutritional support is mandatory for good clinical outcomes. A naso-jejunal tube feeding after acute corrosive injury (>Gr 11b endoscopically) is advocated in our practice. If successful dilatation can be carried out to 8-10 mm a homogenized liquid diet can be allowed orally ensuring adequate calorie and protein intake. Residue at each dilatation could be a marker of the adequacy of previous dilatations. In patients with peptic GOO maintenance of nutrition is not a big problem as response to EBD is prompt.
In one of our studies on benign GOO, we showed that patients with peptic GOO responded uniformly to EBD combined with eradication of H. pylori, and had good long term response as well over a mean follow-up period of 14.04 ± 9.79 months.[31] EBD in peptic GOO had variable results considering the confounding factors of H. pylori eradication, usage of PPI, NSAID abuse, etc. Immediate relief is noted with variable long term responses. Studies that looked for and eradicated H. pylori have reported a good long term response in 70-80% of patients over 9 to 98 months of follow up (Table 2).[28,31,33,37]In the absence of prospective RCTs, the available literature suggests that more than three-fourths of patients with peptic GOO respond to EBD and long term proton pump inhibitor usage may be required to prevent recurrences after H. pylori eradication. Caustic GOO is more difficult to dilate than peptic GOO. Such patients require more sessions and have more recurrences. Solt et al[29] have shown long term response in only one tthird of patients with caustic GOO. We, howwever, observed that out of 41 patients with caustic GOO, [39] patients responded to EBD with a mean (SD) 5.8 ± 2.6 dilations (range 2-13) to achieve the end point of 15 mm. The other 2 patients underwent surgery, one due to perforation and the other due to intractable pain.36 However the mean number of sessions were 2-13 in comparison to peptic GOO which required only 1-3 sessions.[31,36] We have recently shown that early EBD by an expert endoscopist could show better responses in acute caustic GOO.[39] Thirty patients underwent successful dilatations within 2 weeks (14.39 +/- 4.65 days) of acid ingestion to achieve the end point of 15 mm, requiring a median of 9 (range 3-18) dilatations over a period of 7 (range 1.5-16) weeks which persisted for a median 21 (range 3-72) months with no recurrence.
Other causes of GOO like tuberculosis, Crohn’s disease, and pancreatitis do respond to EBD. However they have more recurrences unless the basic disease is managed and adequately treated. EBD in these conditions had beensuccessful as reported by Misra et al[30] in tuberculosis and Kim et al[40] in Crohn’s disease. Anastomotic strictures following vertical band gastroplasty or gastric bypass surgery for morbid obesity have also been successfully dilated with balloon catheters endoscopically.[41]
Complications of EBD
Complications are uncommon with EBD.8 Bleeding and perforation are rarely reported for dilatation less than 15 mm. Increasing the balloon diameter beyond 15 mm is more likely to be associated with perforation. Self limiting pain is common with most EBD. In our study 19.5% of patients with caustic GOO had self-limiting pain during the procedure.[36]
Combination therapy
Intralesional steroids
Augmentation of EBD could be done with intralesional steroids injections and endoscopic incision. Intralesional steroid injections have been shown to inhibit stricture formation by interfering with collagen synthesis, fibrosis and chronic scarring.[42] Triamcinolone prevents the cross linking of collagen that results in scar contracture; so if the scar is stretched and steroid is injected into it, presumably the contracture will not occur.[43] Efficacy of steroids augmenting EBD in GOO has been demonstrated in two studies by Lee et al[44] and Kochhar et al.[45] We reported the use of steroids to augment dilatation in caustic GOO in 3 patients, one of whom was given steroids in the first dilatation itself.[45] All 3 patients had responded to dilatation in 1-2 sessions of injection.
Endoscopic incision
Endoscopic incision followed by EBD had been used and was successful in a few series of caustic GOO. Baron et al[46] used electrosurgical incision using a standard sphincterotomy and Hagiwara et al[47] used needle-knife radial incisions electrosurgically at gastroscopy combined with EBD. We have also used this technique in one of the patients with causticinduced GOO, who had become pregnant while on a dilation programme.[36]
Newer modalities
Placement of biodegradable stents in the enteral tract is an emerging alternative especially in caustic injury. A few case reports have shown efficacy of biodegradable stents in corrosive esophageal strictures.[48,49] Placement of self expanding metal stents (SEMS) is also an upcoming option for benign diseases. The probable role of SEMS is persistent dilatation for an extended period of time than what can be achieved with TTS balloons. However limited experience precludes the routine usage of such devices.
Surgery
Surgery forms the final option for patients presenting with refractory GOO. Most common surgeries for peptic strictures include vagotomy and antrectomy, vagotomy and pyloroplasty, truncal vagotomy and gastrojejunostomy, pyloroplasty and laparoscopic variants. Gastrojejunostomy (Billroth II reconstruction) can be considered in patients with preserved anatomy like peptic GOO, however with altered anatomy, Rouxen- Y jejunostomy would be the preferred option. Placement of a jejunostomy tube at the time of surgery should be considered, as patients with GOO are already malnourished. Also in chronically dilated conditions the stomach is slow to recover the normal rate of emptying. In peptic GOO gastrojejunostomy can be combined with truncal vagotomy and antrectomy. Laparoscopic gastrojejunostomy for the relief of GOO is associated with a smoother and more rapid postoperative recovery and shorter hospital stay compared with open surgery and should become the new gold standard.[50]
References
- Johnson CD. Gastric outlet obstructionmalignant until provenotherwise. Am J Gastroenterol. 1995;90:1740.
- Khullar SK, DiSario JA. Gastric outlet obstruction. GastrointestEndosc Clin N Am. 1996;6:585–603.
- Johnson CD, Ellis H. Gastric outlet obstruction now predictsmalignancy. Br J Surg. 1990;77:1023–4.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outletobstruction. Am J Gastroenterol. 1996;91:1679.
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS.Malignancy is the most common cause of gastric outlet obstructionin the era of H2 blockers. Am J Gastroenterol. 1995;90:1769–70.
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outletobstruction resulting from peptic ulcer disease requiring surgicalintervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg. 2000;191:32–7.
- Misra SP, Dwivedi M, Misra V. Malignancy is the most commoncause of gastric outlet obstruction even in a developing country.Endoscopy. 1998;30:484–6.
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benigngastric outlet obstruction in adults. World J Gastrointest Endosc. 2010;2:29–35.
- Noor MT, Dixit P, Kochhar R, Nagi B, Dutta U, Singh K, Poornachandra KS.NSAIDs-Related pyloroduodenal obstruction and its endoscopic management. Diagn Ther Endosc. 2011;2011:967957.
- Goldstein H, Boyle JD. The saline load test—a bedside evaluation of gastric retention. Gastroenterology. 1965;49:375–80.
- DiSario JA, Fennerty MB, Tietze CC, Hutson WR, Burt RW. Endoscopic balloon dilation for ulcer-induced gastric outlet obstruction. Am J Gastroenterol. 1994;89:868–71.
- Goenka MK, Kochhar R, Ghosh P, Mehta SK. Changing pattern of peptic ulcer in India. An endoscopic study of 1,188 ulcer patients. J Clin Gastroenterol. 1991;13:575–9.
- Subbarao KS, Kakar AK, Chandrasekhar V, Ananthakrishnan N, Banerjee A.Cicatrical gastric stenosis caused by corrosive ingestion. Aust N Z J Surg. 1988;58:143–6.
- Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of corrosive acids. Spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97:702–7.
- Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK.Ingestion of strong corrosive alkalis: spectrum of injury to upper gastrointestinal tract and natural history. Am J Gastroenterol. 1992;87:337–41.
- Chaudhary A, Puri AS, Dhar P, Reddy P, Sachdev A, Lahoti D, et al. Elective surgery for corrosive-induced gastric injury. World J Surg. 1996;20:703–6; discussion 706.
- Goldman G, Tiomny E, Kahn PJ, Somjen D, Halpern Z, Gilat T, et al.Prostaglandin E2 in pyloric stenosis. Arch Surg. 1989;124:724–6.
- Nugent FW, Roy MA. Duodenal Crohn’s disease: an analysis of 89 cases. Am J Gastroenterol. 1989;84:249–54.
- Miner PB, Harri JE, McPhee MS. Intermittent gastric outlet obstruction from a pedunculated gastric polyp. Gastrointest Endosc. 1982;28:219–20.
- Bradley EL 3rd. Complications of chronic pancreatitis. Surg Clin North Am. 1989;69:481–97.
- Aranha GV, Prinz RA, Greenlee HB, Freeark RJ. Gastric outlet and duodenal obstruction from inflammatory pancreatic disease. Arch Surg. 1984;119:833.
- Green ST, Drury JK, McCallion J, Erwin L. Carcinoid tumour presenting as recurrent gastric outlet obstruction: a case of longterm survival. Scott Med J. 1987;32:54–5.
- Graham DY. Ulcer complications and their nonoperativetreatment.In: Sleisenger M, Fordtran J (eds). Gastrointestinal Disease. Philadelphia, PA: WB Saunders; 1993.p.698.
- Soll AH, Graham DY. Peptic ulcer disease. In: Yamada T, editor. Yamada Textbook of Gastroenterology. 5th edition. Oxford, UK: Wiley-Blackwell Publishing Ltd; 2009.p.936–81.
- Benjamin SB, Cattau EL, Glass RL. Balloon dilation of the pylorus: therapy forgastric outlet obstruction. Gastrointest Endosc. 1982;28:253–4.
- Benjamin SB, Glass RL, Cattau EL Jr, Miller WBl. Preliminary experience withballoon dilation of the pylorus. Gastrointest Endosc. 1984;30:93–5.
- Lau JY, Chung SC, Sung JJ, Chan AC, Ng EK, Suen RC, Li AK. Through-the-scope balloon dilation for pyloric stenosis: longterm results. Gastrointest Endosc. 1996;43:98–101.
- Boylan JJ, Gradzka MI. Long-term results of endoscopic balloon dilatation for gastric outlet obstruction. Dig Dis Sci. 1999;44:1883–6.
- Solt J, Bajor J, Szabó M, Horváth OP. Long-term results of balloon catheter dilation for benign gastric outlet stenosis. Endoscopy. 2003;35:490–5.
- Misra SP, Dwivedi M. Long-term follow-up of patients undergoing ballon dilation for benign pyloric stenoses. Endoscopy. 1996;28:552–4.
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol. 2004;19:418–22.
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol. 1996;91:987–90.
- Cherian PT, Cherian S, Singh P. Long-term follow-up of patients with gastric outlet obstruction related to peptic ulcer disease treated with endoscopic balloon dilatation and drug therapy. Gastrointest Endosc. 2007;66:491–7.
- Kozarek RA, Botoman VA, Patterson DJ. Long-term follow-up in patients who have undergone balloon dilation for gastric outlet obstruction. Gastrointest Endosc. 1990;36:558–61.
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc. 1995;41:15–7.
- Kochhar R, Dutta U, Sethy PK, Singh G, Sinha SK, Nagi B, et al. Endoscopic balloon dilation in caustic-induced chronic gastric outlet obstruction. Gastrointest Endosc. 2009;69:800–5.
- Lam YH, Lau JY, Fung TM, Ng EK, Wong SK, Sung JJ, et al. Endoscopic balloon dilation for benign gastric outlet obstruction with or without Helicobacter pylori infection. Gastrointest Endosc. 2004;60:229–33.
- Griffin SM, Chung SC, Leung JW, Li AK. Peptic pyloric stenosis treated by endoscopic ballon dilatation. Br J Surg. 1989;76:1147–8
- Kochhar R, Poornachandra KS, Dutta U, Agrawal A, Singh K. Early endoscopic balloon dilation in caustic-induced gastric injury.Gastrointest Endosc. 2010;71:737–44.
- Kim JH, Shin JH, Di ZH, Ko GY, Yoon HK, Sung KB, et al.Benign duodenal strictures: treatment by means of fluoroscopically guided balloon dilation. J Vasc Interv Radiol. 2005;16:543–8.
- Sataloff DM, Lieber CP, Seinige UL. Strictures following gastric stapling for morbid obesity. Results of endoscopic dilatation. Am Surg. 1990;56:167–74.
- Ashcraft KW, Holder TM. The experimental treatment of esophageal strictures by intralesional steroid injections. J Thorac Cardiovasc Surg. 1969;58:685–91.
- Ketchum LD, Smith J, Robinson DW, Masters FW. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1966;38:209–18.
- Lee M, Kubik CM, Polhamus CD, Brady CE 3rd, Kadakia SC. Preliminary experience with endoscopic intralesional steroid injection therapy for refractory upper gastrointestinal strictures. Gastrointest Endosc. 1995;41:598–601.
- Kochhar R, Sriram PV, Ray JD, Kumar S, Nagi B, Singh K. Intralesional steroid injections for corrosive induced pyloric estenosis. Endoscopy. 1998;30:734–6.
- Baron B, Gross KR. Successful dilation of pyloric stricture resistant to balloon dilation with electrocautery using asphincterotome. J Clin Gastroenterol. 1996;23:239–41.
- Hagiwara A, Sonoyama Y, Togawa T, Yamasaki J, Sakakura C, Yamagishi H. Combined use of electrosurgical incisions and balloon dilatation for the treatment of refractory postoperative pyloric stenosis. Gastrointest Endosc. 2001;53:504–8.
- Zhang C, Yu JM, Fan GP, Shi CR, Yu SY, Wang HP, Ge L, Zhong WX.The use of a retrievable self-expanding stent in treating childhood benign esophageal strictures. J Pediatr Surg.2005;40:501–4.
- Vandenplas Y, Hauser B, Devreker T, Urbain D, Reynaert H. A degradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. Endoscopy. 2009;41:E73.
- Al-Rashedy M, Dadibhai M, Shareif A, Khandelwal MI, Ballester P, Abid G, et al. Laparoscopic gastric bypass for gastric outlet obstruction is associated with smoother, faster recovery and shorter hospital stay compared with open surgery. J Hepatobiliary Pancreat Surg. 2005;12:474–8.
|