Emad Kandil1, Mohamed Abdel Khalek1, Obai Abdullah1, Salima Haque2, Angela Bohlke2, Bernard Jaffe1
Division of Endocrine and Oncologic Surgery,
Department of General Surgery,1
Department of Pathology,2
Tulane University School of Medicine,
1430 Tulane Ave, SL-22 New Orleans,
LA 70112-2699
Corresponding Author:
Dr. Emad Kandil
Email: ekandil@tulane.edu
48uep6bbphidvals|453 48uep6bbph|2000F98CTab_Articles|Fulltext Granular cell tumor is relatively rare soft tissue tumor that can
present anywhere in the body. It commonly occurs in oral
cavities and subcutaneous tissues, but is uncommon in the
colon and rectum.[1, 2] This usually benign tumor appears as a
submucosal nodule, measuring less than 2 cm in diameter, and
is often found incidentally during colorectal examinations.[2, 3]
Case report
We report a healthy 52 years old African American patient who
had a screening colonoscopy. He has a strong family history
of colon cancer in his mother and two of his siblings.
Colonoscopy revealed evidence of a 3 cm submucosal caecal
mass that was not amenable to endoscopic resection due to
subserosal involvement.
CT scan of abdomen and pelvis showed no evidence of
any other associated mass or metastasis. The patient
underwent a laparoscopic right hemicolectomy with robotic
assistance. His postoperative course was uneventful and the patient was discharged home after three days. Histological
examination of the resected mass revealed a poorly
circumscribed collection of pale eosinophilic cells extensively
infiltrating throughout the muscularis propria as well as focal
areas of the submucosa. The cells varied in shape from round
to spindled and had marked cytoplasmic granularity. The nuclei
had varied appearances, ranging from small and dark to slightly
larger and vesicular.No mitosis was noted. Periodic acid-Schiff
staining accentuated the cytoplasmic granules (Figure 1).
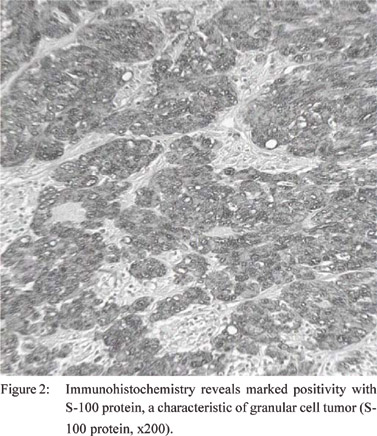
Immunohistochemistry demonstrated marked positivity with
S-100 protein, CD68, and neuron-specific enolase and negativity
with neurofilament (Figure 2). This was consistent with the
characteristic staining profile of a granular cell tumor.

Discussion
The gastrointestinal tract is an unusual location for granular
cell tumor.[4] In contrast to the present case, it usually involves
submucosal area and measures less than two cm.[2,3] It is
uncommonly detected in the muscle layer and in subserosal
areas.[2, 5, 6] Granular cell tumor has a benign clinical course and
an incidence of local recurrence in 5-10% cases after surgical
resection.[1,7] It could seldom be diagnosed based on
macroscopic and endoscopic examination due to both its small
size and its shape resembling a diminutive polyp.[1]
People with two first-degree relatives who have,
respectively, a colorectal cancer and a colonic adenoma should
be offered screening colonoscopy every three to five years
beginning at the age of 40.8 Granular cell tumor rarely gives rise
to diagnostic problems because of its characteristic histologic
picture.[4] These tumors seem to derive from Schwann cells,
even if the earlier reports considered them to be of muscular
origin.[9] Patients who are diagnosed with a granular cell tumor
of uncertain malignant potential may benefit from preoperative
radiologic evaluation because occult metastatic disease when
identified, alters the surgical approach and possibly affect the
long-term outcome.[10] The final diagnosis of granular cell tumor
depends upon histo-pathological findings.[11]Since this tumor
is considered to be usually benign, endoscopic removal has
been recommended for management of colonic granular cell
tumor[2,9,12,13] especially when endoscopic ultrasonography
shows that the tumor is smaller than 2 cm and is well separated
from the muscularis propria.[2,13,14] However, as in the present
case, surgical resection should be considered for those cases
in which endoscopic removal is not feasible and a limited
surgical resection is indicated in order to obtain a clear
diagnosis.9 In addition, surgical resection may become the
treatment for a benign granular cell tumor because of the
misinterpretation of the biopsied lesion.[15]
References
- Lack EE, Worsham GF, Callihan MD, Crawford
BE, Klappenbach S, Rowden G, et al. Granular cell tumor: a
clinicopathologic study of 110 patients. J Surg Oncol.
1980;13:301–16.
- Yasuda I, Tomita E, Nagura K, Nishigaki Y, Yamada O, Kachi H.
Endoscopic removal of granular cell tumors. Gastrointest Endosc.
1995;41:163–7.
- Melo CR, Melo IS, Schmitt FC, Fagundes R, Amendola D.
Multicentric granular cell tumor of the colon: report of a patient
with 52 tumors. Am J Gastroenterol. 1993;88:1785–7.
- Yamada T, Fujiwara Y, Sasatomi E, Nakano S, Tokunaga O.
Granular cell tumor in the ascending colon. Intern Med.
1995;34:657–60.
- Yamaguchi K, Maeda S, Kitamura K. Granular cell tumor of the
stomach coincident with two early gastric carcinomas. Am J
Gastroenterol. 1989;84:656–9.
- Uzoaru I, Firfer B, Ray V, Hubbard-Shepard M, Rhee H.
Malignant granular cell tumor. Arch Pathol Lab Med.
1992;116:206–8.
- Armin A, Connelly EM, Rowden G. An immunoperoxidase
investigation of S-100 protein in granular cell myoblastomas:
evidence for Schwann cell derivation. Am J Clin Pathol.
1983;79:37–44.
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow
CD, et al. Colorectal cancer screening: clinical guidelines and
rationale. Gastroenterology. 1997;112:594–642.
- Rossi GB, de Bellis M, Marone P, De Chiara A, Losito
S, Tempesta A. Granular cell tumors of the colon: report of a case
and review of the literature. J Clin Gastroenterol. 2000;30:197–
9.
- Jardines L, Cheung L, LiVolsi V, Hendrickson S, Brooks JJ.
Malignant granular cell tumors: report of a case and review of the
literature. Surgery. 1994;116:49–54.
- Sohn DK, Choi HS, Chang YS, Huh JM, Kim DH, Kim DY, et
al. Granular cell tumor of colon: report of a case and review of
literature. World J Gastroenterol. 2004;10:2452–4.
- Endo S, Hirasaki S, Doi T, Endo H, Nishina T, Moriwaki T, et
al. Granular cell tumor occurring in the sigmoid colon treated by
endoscopic mucosal resection using a transparent cap (EMR-C).
J Gastroenterol. 2003;38:385–9.
- Kawamoto K, Yamada Y, Furukawa N, Utsunomiya T, Haraguchi
Y, Mizuguchi M, et al. Endoscopic submucosal tumorectomy
for gastrointestinal submucosal tumors restricted to the
submucosa: a new form of endoscopic minimal surgery.
Gastrointest Endosc. 1997;46:311–7.
- Kajiyama T, Hajiro K, Sakai M, Inoue K, Konishi Y, Takakuwa
H, et al. Endoscopic resection of gastrointestinal submucosal
lesions: a comparison between strip biopsy and aspiration
lumpectomy. Gastrointest Endosc. 1996;44:404–10.
- Cha JM, Lee JI, Joo KR, Choe JW, Jung SW, Shin HP, et al.
Granular cell tumor of the descending colon treated by endoscopic
mucosal resection: a case report and review of the literature. J
Korean Med Sci. 2009;24:337–41.
|