Amit Kumar Dutta, Manoj Kumar Sahu, Sajith Kattiparambil Gangadharan, Ashok Chacko
Department of Gastrointestinal Sciences,
Christian Medical College & Hospital, Vellore – 632004,
Tamil Nadu, India
Corresponding Author:
Dr. Ashok Chacko
Email: gastro@cmcvellore.ac.in
Abstract
Background: Distinguishing Crohn’s disease (CD) from intestinal tuberculosis (ITB) is clinically challenging but important for prognostication and patient management.
Methods: Patients with diagnosis of CD and ITB were prospectively enrolled in the study from January 2006 to October 2007. The patients were followed up for further 15 months to ascertain that the diagnosis had not changed. Clinical, laboratory, serological [IgG anti Saccharomyces cerevisiae antibody (ASCA)], endoscopic and histologic features were compared between the ITB and CD patients. The ASCA titers were estimated in 100 healthy controls. Patients were diagnosed as ASCA positive when their ASCA titers were three standard deviations above mean of controls.Results: Thirty patients with CD (age 33.9 + 15.2 years, 70% males) and thirty with ITB (age 35.1 + 12.2years, 53.3% males) were included in the study. Features commoner in CD were longer duration of symptoms (p<0.001), blood mixed stool (p=0.006), presence of longitudinal ulcers (p=0.005) and skip lesions (p=0.008) on colonoscopy and more number of colonic segments involved (p=0.004). Anorexia was commoner in ITB patients (p=0.008). Positive ASCA was commoner in CD (30%) than ITB (10%) but did not reach statistical significance (p=0.1).
Conclusions: A combined evaluation of clinical features, endoscopy, histology and response to treatment is the key to differentiate between CD and ITB.
|
48uep6bbphidvals|440 48uep6bbph|2000F98CTab_Articles|Fulltext The last two decades has seen the emergence of Crohn’s
disease (CD) in developing countries like India where intestinal
tuberculosis (ITB) is prevalent as well.[1] Distinguishing CD
from ITB is often challenging as both diseases have similar
radiological, endoscopic and histologic features.[2] Since
treatment and prognosis of the two conditions are different, it
is crucial to diagnose them correctly.
Tests for evaluation of patients with suspicion of CD or
ITB include abdominal imaging, serological tests, endoscopy
and histology. Results of individual investigations (except for
presence of AFB or caseating granuloma for tuberculosis) are
often insufficient for diagnosis. Diagnosis is often made after
a combined evaluation of above investigations and response
to therapy.[2] In this prospective study we report the role of clinical features, serology, endoscopy and histology in
distinguishing CD from ITB.
Methods
Patients diagnosed to have CD and ITB at our institute were
prospectively enrolled in the study between January 2006 and
October 2007. Thirty patients with CD and thirty with ITB
were recruited. The diagnosis of Crohn’s disease was made
based on a combination of clinical, radiological, endoscopic
and histological features suggested by European evidence
based consensus on the diagnosis and management of Crohn’s
disease.[3]Intestinal tuberculosis was diagnosed in the presence
of any of the following features: (I) Intestinal mucosal biopsy
showing - a) AFB positive on histopathology or culture, and/
or b) caseating granulomas, and/or c) large or confluent
granulomas (II) Response to treatment.[4]
Clinical, investigation and treatment data were
systematically recorded on a pre-designed proforma.
Colonoscopy and terminal ileoscopy (if possible) (Olympus,
CF 150L) was performed on the study subjects and gross
findings were recorded. Multiple mucosal biopsy specimens
were obtained from different segments of colon and terminal
ileum during the scopy. Upper gastrointestinal endoscopy
(Olympus, GIF 150) and biopsy from duodenum (D2) and
stomach (antrum and incisura) were obtained in all CD patients
irrespective of gross findings to assess extent of disease.
Evaluation of anti saccharomyces cerevisae antibody (ASCA)
was done using the IgG ASCA ELISA kits (AIDA diagnostics,
Germany). ASCA recognizes specific mannan, a component of
the outer wall of yeast.[5] Serum samples were incubated in the
microplates coated with specific antigen. Patient’s antibodies,
if present in the specimen, bind to the antigen. The unbound
fraction was washed off in subsequent steps. Afterwards, antihuman
immunoglobulins conjugated to horseradish peroxidase
(conjugate) were incubated and reacted with the antigenantibody
complex of the samples in the microplates. Unbound
conjugate was washed off. Addition of 3,5,3',5'-
tetramethylbenzidine (TMB)-substrate generated an enzymatic
colorimetric (blue) reaction and the absorbance was read at
450 nm within 30 minutes. The optical density (OD) of each
calibrator (y axis) was plotted against the corresponding
concentration values in U/ml (x axis). From this plot the
antibody concentration of each sample was calculated by
finding out the concentration corresponding to their respective
OD values. ASCA levels were assessed in 100 healthy controls.
The test was considered positive in patients if OD values were
3 standard deviation above mean value of controls.
After the diagnosis of CD or ITB was made, the patients
were followed up for a further 15 months to assess response to
therapy and to confirm that the diagnosis had not changed.
Clinical profile, serology, endoscopy and histopathology were
compared between the two groups of patients to assess the
ability of the above to differentiate Crohn’s disease from
intestinal tuberculosis. The study was approved by the
institutional review board.
Data is presented as mean with standard deviation for
normally distributed continuous variables and as median with range for non-normally distributed continuous variables.
Categorical data is presented as proportions. For comparing
categorical variables (including ASCA) chi square test was
used. For comparing continuous variables with normal
distribution, t test was used. For continuous variable with nonnormal
distribution, Mann-Whitney’s U test was used. A p
value of <0.05 was considered significant. The statistical
analysis was done using SPSS software for windows version
11.0.
Results
We selected 67 probable patients for our study of whom 60 (30
CD and 30 ITB) fulfilled the inclusion criteria. Four patients
with suspected ITB and 3 with suspected CD were excluded
due to insufficient diagnostic criteria. At baseline, 5 patients
with CD (included in the study) had history of receiving ATT
in past. Demographic and clinical features in patients with CD
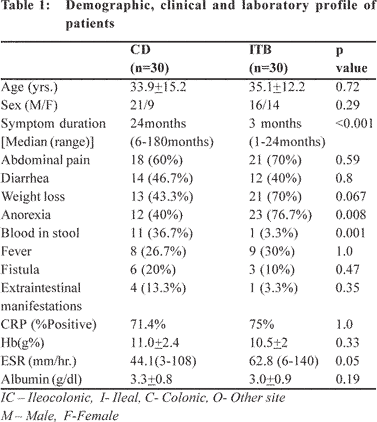
and ITB are shown in (Table 1). There was no significant
difference in age, sex and geographical location in patients
with CD and ITB. The median duration of symptoms in patients
with ITB was 3 months (range 1 month to 2 years) while it was
2 years (range 6 months to 15 years) in patients with CD, the
difference being statistically significant (p <0.001). Dull aching
and poorly localized abdominal pain was the commonest
symptom in patients with CD (60%) and ITB (70%). Blood mixed
with stool was seen more often in patients with CD (p=0.006).
Anorexia (p=0.008) and weight loss (p=0.067) were seen more
often in ITB. Diarrhoea and fever were similar in both groups.
Clinical examination of abdomen showed right iliac fossa mass
in two patients with CD. In patients with ITB right iliac fossa
mass was revealed in two patients, hepatosplenomegaly in one, hepatomegaly in one and cervical lymphadenopathy in two patients.
According to the Vienna classification, the disease location
in CD patients was L1 (ileal) in 13.3%, L2 (colonic) in 23.3%, L3
(ileocolonic) in 60% and L4 (upper gastrointestinal) in 3.3%.
Although upper gastrointestinal lesion was seen in 5 patients
(on endoscopy and/or histology), 4 of them had major lesions
in ileum and/or colon and hence were not classified as L4. Two
patients with CD had internal fistula (jejunocolic and
colovesical), 4 had fistula-in-ano and two had anal fissure.
Extraintestinal manifestations were present in 4 patients – 3
had arthralgia, 1 had pyoderma gangrenosum and 3 had oral
ulcers. Thirteen patients underwent surgery for their disease –
5 had small bowel surgery for strictures, 7 had right
hemicolectomy for ileocolonic disease and 1 had fistulectomy
for perianal fistula. The disease location in ITB patients was
ileocolonic in 53.3%, ileal in 3.3%, colonic in 33.3% and upper
gastrointestinal in 10%. Among patients with ITB two patients
had internal fistula (duodenocolic and jejunocolic) and 1 had
fistula-in-ano. Extraintestinal manifestation was present in only
1 patient who had arthralgia. Three patients underwent surgery
for their disease – 1 had small bowel surgery for strictures and
other two had partial colonic resections as treatment of internal
fistulas. Patients with CD underwent surgery significantly more
often compared to ITB patients (p=0.007). Lab investigations
revealed low albumin (<3.5 g/dl in 38 patients), high ESR (>30 in 40 patients) and borderline low hemoglobin (<11.0 g% in 32
patients) in most patients. ESR was significantly higher in ITB
group compared to CD (p=0.05).
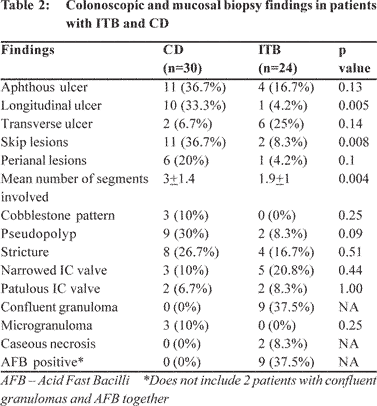
All patients with CD and 24 patients with ITB underwent
colonoscopy. The other 6 patients with ITB had radiological
evidence of intestinal involvement with evidence of
tuberculosis at extraintestinal sites. The colonoscopy and
mucosal biopsy findings are shown in (Table 2). Colonoscopy
showed lesions in twenty five (83%) patients with CD and
twenty three patients with ITB (95.8%). Presence of skip lesions
(p=0.008), longitudinal ulcers (p=0.005), and multiple colonic
segment involvement (p=0.004) were commoner in CD than in
ITB. Other colonoscopic findings were not helpful in
differentiating CD from ITB. Three patients with CD had UGI
involvement on endoscopy (gastric erosions in 2 and duodenal
nodularity in 1) and five had noncaseating granulomas on
histology (4 in stomach and 1 in duodenum).
Histological features suggestive of CD were seen in 90%
of CD patients and these included crypt architecture
abnormalities, mononuclear infiltration, granulomas, transmural
inflammation, segmental distribution of the lesion and patchy
and focal inflammation. Remaining three patients had nonspecific
histological features and in them, clinical and
radiological features along with response to therapy formed
the basis of diagnosis. All patients with CD showed response
to treatment but 6 of them had a relapse during follow up.
Histological features suggestive of ITB were seen in 20 of the 24 ITB patients undergoing colonoscopy and these included
large or confluent granulomas (n=9, 37.5%), caseating
granulomas (n=2, 8.3%), and presence of AFB in tissue section
or culture of tissue (n=9, 37.5%). The ITB patients with caseation
(n=2) or AFB (n=9) had discrete (non-confluent) granulomas
seen on histology. Among the 9 patients with confluent
granulomas, 2 had AFB. Of the 30 ITB patients, 27 responded
to ATT. Among the remaining 3 (all had AFB on histology), 1
expired after 1 month of therapy and 2 had no response to 9
months of ATT.
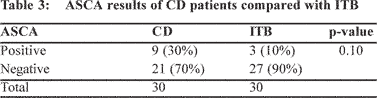
ASCA ELISA was tested on 100 controls to establish the
normal “cut-off” antibody levels in the population. The mean
optical density value in controls plus 3 standard deviation was
taken as “cut-off” value and patients with values above this
level (equivalent to 7 U/ml) were considered to have a positive
ASCA test. 30% of patients with CD had ASCA positive as
compared to 10% of patients with ITB (p=0.1) (Table 3). The
specificity of ASCA was 90% in differentiating CD from ITB.
The occurrence of stricturing and fistulising disease, small
bowel disease, history of surgery, marker of disease activity
(CRP, ESR, Harvey Bradshaw Score >4) and clinical course
were not significantly different between the CD patients who
were ASCA positive and those who were ASCA negative.
However, the small number of patients makes meaningful
comparison difficult.
Discussion
The study was aimed at assessing distinguishing features
between CD and ITB in a prospectively recruited cohort. About
35-45% of patients with CD in India are initially diagnosed to
have ITB which aptly highlights the difficulty in distinguishing
between the two diseases.[6,7] The rising incidence of CD in
India further compounds this problem and calls for development
of effective tools to differentiate the two conditions.[1] Both the
diseases are common in the fourth decade of life and abdominal
pain and weight loss are seen in up to two-third of the cases.[4,6]
A large case series of ITB (n=173) and another one on CD
(n=182) from India suggests that ITB patients have more
frequent fever and shorter duration of symptoms (<1 year).[4,6] CD patients are usually symptomatic for years and diarrhea
and bleeding per rectum are more common.6,7 Symptom duration was shorter and anorexia was more common in our
ITB patients. Blood in stool was commoner in CD patients
while rest of the clinical features were comparable. Amarapurkar
et al have reported anemia to be frequent in CD patients and
ESR similar in patients with CD and ITB.[7] Our ITB patients had
higher ESR than CD patients. In our study, lab features were
not helpful in differentiating the two diseases.
Serological tests including ASCA and pANCA are
commonly used in western countries to distinguish CD from
ulcerative colitis.[8] ASCA is commoner in CD and pANCA in
ulcerative colitis.8 Since in India we are more often faced with
problems of distinguishing CD from ITB, it is logical to study
the role of serological tests to differentiate between them. Three
studies have been done in India on utility of ASCA but none
showed any significant difference in prevalence between CD
and ITB patients.[7,9,10] However, these reports have not
commented on establishing local positive cut-offs of ASCA.
We analyzed the serum of 100 healthy controls to establish the
cut-off value of ASCA in our population. Using this cut-off,
30% of our patients with CD and 10% of patients with ITB were
ASCA positive but the difference failed to reach statistical
significance. Interestingly, the ASCA prevalence in CD in other
Indian studies has ranged from 40-60% as compared to 30% in
our series.[7,9,10] The use of cut-off value of ASCA established
for our own population in this study may account for this
difference. However, ASCA did have a good specificity in
distinguishing CD from ITB. Eight of our nine CD patients
who had ASCA positive had ileal involvement suggesting that
ASCA positivity may point to small bowel involvement in CD.
Radiological investigations provide useful clues to
diagnosis but their major role is in assessing the extent of the
disease.[2] Since we used both barium studies and CT abdomen
for this purpose, comparison of any single modality to
differentiate the two diseases was not performed due to small
number of patients. Studies comparing imaging findings in CD
and ITB are few[11,12] and most describe findings in either a group
of ITB patients or CD patients.[13,14] Asymmetric thickening of
colonic wall, larger lymph nodes (>1cm), necrotic lymph nodes
and ascites on CT are suggestive of ITB.2 Symmetrical bowel
wall thickening, smaller (<1cm) lymph nodes and bowel loop
displacement by fibrofatty changes are suggestive of CD.[2]
Endoscopy plays an important role in diagnosis.[7,15] Apart
from visual inspection, mucosal biopsy can be obtained for
histopathology, culture and molecular tests. Presence of
mucosal ulcers and involvement of ileocecal region are common
in both diseases. Colonoscopy studies show that CD patients usually have longitudinal ulcers, cobblestone appearance of
mucosa and anorectal involvement.[16] A patulous ileocecal
valve, transverse ulcer and involvement of lesser number of
colonic segments are commoner in ITB.[7,16] None of these were
discriminatory in our patients. Presence of AFB in biopsy
samples on histology or on culture is diagnostic of tuberculosis
but was present only in a small number of our cases. Granulomas in endoscopic biopsy samples are seen in 20-30%
of CD and 50-70% of ITB patients.[6,7,17,18] Previous studies done
at our center by Pulimood et al showed that granulomas in ITB
and CD have different morphology.[19,20] Granulomas in ITB are
multiple (mean number of granulomas per section: 5.35), large
(mean widest diameter: 193 micron), confluent and often with
caseating necrosis. In CD granulomas were infrequent, small
and poorly organized. In addition to microscopic examination,
tissue samples can be subjected to PCR tests. Conventional
PCR is done after extracting nucleic acids from tissue followed
by amplification reactions. In a report on 60 patients with ITB
and 20 with CD, PCR was positive in 21.6% of ITB patients vs.
5% CD patients.[21] Another PCR technique called the ‘in-situ
PCR’ does not require nucleic acid extraction from tissues.
The targeted DNA sequence is amplified in intact cells. A study
done at our center on 20 patients with ITB and CD each showed
it to be positive in 30% and 5% patients respectively.[22] What
is apparent from these data is that PCR has a good specificity
but poor sensitivity. Apart from tissue PCR, fecal sample PCR
testing has also been studied at our center in ITB patients and
controls and the results are encouraging.[23]
In conclusion, differentiating CD from ITB continues to be
a challenging problem. Presently, a combination of clinical
features, endoscopy, histology, radiology and response to
treatment continues to be the key to differentiate these two
conditions. We need to continue to develop new tests to help
clinicians differentiate between the two conditions.
Acknowledgements
We thank Dr. Gagandeep Kang, Mrs. Sheela Roy and other
staff members in the Microbiology wing of the department of
Gastrointestinal sciences who performed the ASCA test on
serum of patients and controls.
References
- Desai HG, Gupte PA. Increasing incidence of Crohn’s disease in
India: is it related to improved sanitation? Indian J Gastroenterol.
2005;24:23–4.
- Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal
tuberculosis from Crohn’s disease: a diagnostic challenge. Am J
Gastroenterol. 2009;104:1003–12.
- Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas
L, Geboes K, et al. European evidence based consensus on the
diagnosis and management of Crohn’s disease: definitions and
diagnosis. Gut. 2006;55 Suppl 1:i1–15.
- Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha
P, Pramanik S, et al. Gastrointestinal luminal tuberculosis:
establishing the diagnosis. J Gastroenterol Hepatol.
2004;19:1240–6.
- Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros
A. Serologic testing with ANCA, ASCA, and anti-OmpC in
children and young adults with Crohn’s disease and ulcerative
colitis: diagnostic value and correlation with disease phenotype.
Am J Gastroenterol. 2004;99:2235–41.
- Das K, Ghoshal UC, Dhali GK, Benjamin J, Ahuja V, Makharia
GK. Crohn’s disease in India: a multicenter study from a country
where tuberculosis is endemic. Dig Dis Sci. 2009;54:1099–107.
- Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s
disease in India where tuberculosis is widely prevalent. World J
Gastroenterol. 2008;14:741–6.
- Barahona-Garrido J, Sarti HM, Barahona-Garrido MK,
Hernández-Calleros J, Coss-Adame E, Garcia-Saenz SM, et al.
Serological markers in inflammatory bowel disease: a review of
their clinical utility. Rev Gastroenterol Mex. 2009;74:230–7.
- Makharia GK, Sachdev V, Gupta R, Lal S, Pandey RM. Anti-
Saccharomyces cerevisiae antibody does not differentiate between
Crohn’s disease and intestinal tuberculosis. Dig Dis Sci.
2007;52:33–9.
- 1Ghoshal UC, Ghoshal U, Singh H, Tiwari S. Anti-
Saccharomyces cerevisiae antibody is not useful to differentiate
between Crohn’s disease and intestinal tuberculosis in India. J
Postgrad Med. 2007;53:166–70.
- Makanjuola D. Is it Crohn’s disease or intestinal tuberculosis?
CT analysis. Eur J Radiol. 1998;28:55–61.
- Boudiaf M, Zidi SH, Soyer P, Lavergne-Slove A, Kardache
M, Logeay O, et al. Tuberculous colitis mimicking Crohn’s
disease: utility of computed tomography in the differentiation.
Eur Radiol. 1998;8:1221–3.
- Balthazar EJ, Gordon R, Hulnick D. Ileocecal tuberculosis: CT
and radiologic evaluation. AJR Am J Roentgenol. 1990;154:499–
503.
- Sinan T, Sheikh M, Ramadan S, Sahwney S, Behbehani A. CT features in abdominal tuberculosis: 20 years experience. BMC Med Imaging. 2002;2:3.
- Shah S, Thomas V, Mathan M, Chacko A, Chandy G, Ramakrishna
BS, et al. Colonoscopic study of 50 patients with colonic
tuberculosis. Gut. 1992;33:347–51.
- Lee YJ, Yang SK, Byeon JS, Myung SJ, Chang HS, Hong SS, et
al. Analysis of colonoscopic findings in the differential diagnosis
between intestinal tuberculosis and Crohn’s disease. Endoscopy.
2006;38:592–7.
- Kim KM, Lee A, Choi KY, Lee KY, Kwak JJ. Intestinal
tuberculosis: clinicopathologic analysis and diagnosis by
endoscopic biopsy. Am J Gastroenterol. 1998;93:606–9.
- Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical,colonoscopic, and histological profile of colonic tuberculosis in a
tertiary hospital. Endoscopy. 2005;37:351–6.
- Pulimood AB, Ramakrishna BS, Kurian G, Peter S, Patra
S, Mathan VI, et al. Endoscopic mucosal biopsies are useful in
distinguishing granulomatous colitis due to Crohn’s disease from
tuberculosis. Gut. 1999;45:537–41.
- Pulimood AB, Peter S, Ramakrishna B, Chacko A, Jeyamani
R, Jeyaseelan L, et al. Segmental colonoscopic biopsies in the
differentiation of ileocolic tuberculosis from Crohn’s disease. J
Gastroenterol Hepatol. 2005;20:688–96.
- Amarapurkar DN, Patel ND, Amarapurkar AD, Agal S, Baigal
R, Gupte P. Tissue polymerase chain reaction in diagnosis of
intestinal tuberculosis and Crohn’s disease. J Assoc Physicians
India 2004;52:863–7.
- Pulimood AB, Peter S, Rook GW, Donoghue HD. In situ PCR
for Mycobacterium tuberculosis in endoscopic mucosal biopsy
specimens of intestinal tuberculosis and Crohn disease. Am J
Clin Pathol. 2008;129:846–51.
- Balamurugan R, Venkataraman S, John KR, Ramakrishna BS.
PCR amplification of the IS6110 insertion element of
Mycobacterium tuberculosis in fecal samples from patients with
intestinal tuberculosis. J Clin Microbiol. 2006;44:1884–6.
|