48uep6bbphidvals|438
48uep6bbph|2000F98CTab_Articles|Fulltext
Hydatid disease is a zoonotic infection caused by larval forms
of Echinococcus granulosus and is endemic in the
Mediterranean area, Middle East, South America, India,
northern China and Australia. Cystic hydatid disease usually
affects the liver (50–70%) and less frequently the lung, spleen,
kidney, bone, and brain.[1] Hepatic echinococcal cysts generally
remain asymptomatic until their expanding size or their spaceoccupying effect result in symptoms. Most common
presentation is abdominal pain or a palpable mass in the right
upper quadrant. Rupture of or episodic leakage from a hydatid
cyst may produce fever, pruritis, urticaria, eosinophilia, or
anaphylaxis. Infection of the cyst can facilitate the development
of liver abscesses and mechanical local complications such as
mass effect on bile ducts and vessels that can induce
cholestasis, portal hypertension and Budd-Chiari
syndrome.[2, 3] In addition, the presence of daughter cysts in an
older cyst represents a significant risk of recurrence after
surgery. Therefore, treatment of liver hydatid cysts is
considered mandatory in symptomatic cysts and is
recommended in viable cysts because of the risk of severe
complications.[1]
Treatment of hepatic hydatid is based on ultrasound staging
and considerations of the size, location and manifestations of
cysts, and the overall health of the patient. There are several
classification schemes for liver hydatid cysts based on their
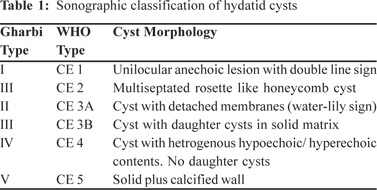
ultrasound appearance; the initial classification by Gharbi et al
and the WHO Informal Working Group on Echinococcosis
(IWGE) classification are the most commonly preferred.[4] Hassen Gharbi classified hepatic hydatid into five types based
on sonographic appearance.[5] WHO classification is almost the
same as Gharbi’s, with Gharbi type II corresponding to CE 3A
of the WHO classification, and vice versa. However, there are
two important additions in the WHO classification: the
predominantly solid cyst with daughter cysts, which was not
explicitly included in Gharbi’s classification, has found a place
in the CE 3 slot, and the types are now grouped according to
their biological activity (Table 1). This has important
consequences for treatment decisions.[6]
For many years surgery was the only available treatment
for hepatic hydatid cysts and was associated with high
morbidity, mortality, and relapse rates of 32%, 8%, and 20%,
respectively.[6] The failure rate for medical management of
hydatid cysts with benzimidazole compounds (i.e., albendazole
and mebendazole) used alone approaches 25%, with most cases
of relapse occurring within 2 years of cessation of therapy.[7] In
recent years percutaneous, minimally, invasive treatment of
hepatic hydatid cysts has developed into an attractive
alternative to surgery and benzimidazole derivatives for certain
cyst stages.[6] It has been tried with considerable success in
types I and II, with encouraging long term results.[8] However
reports on percutaneous treatment of type III cysts are limited.
At our institution surgery is still the mainstay of treatment of
Gharbi type III. We are presenting our experience with treating
these patients using minimally invasive techniques in a select
group of patients who refused surgery.
Methods
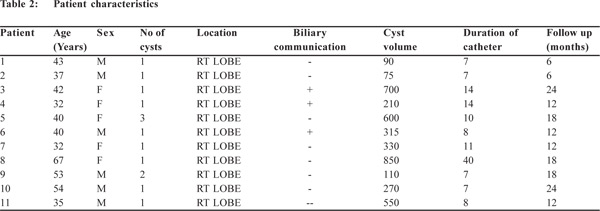
From March 1994 to November 2007, 11 patients with 13 Gharbi
type III (WHO type CE 2 and CE 3B) hepatic hydatid cysts
were enrolled for our study. This study was conducted in
Department of Radiodiagnosis, Sanjay Gandhi Post-graduate
Institute of Medical Sciences, Lucknow (UP), India. There
were 6 female and 5 male patients; age ranging from 32 – 67 yrs
(mean age 43 yrs). The chief presenting complaints were right upper quadrant (RUQ) pain (n = 9), RUQ mass (n = 7), and
epigastric discomfort (n = 8). In 2 patients the cysts were
incidentally detected during imaging for unrelated complaints.
No patient had prior surgical intervention. The diagnosis of
heterogeneous hepatic hydatid cysts was based on
characteristic cyst morphology on cross sectional imaging,
ultrasonography (USG) (Figure 1) and computerized
tomography (CT) (Figure 2) and indirect hemagglutination
(IHA) test. All heterogeneous hepatic hydatid cysts were
located in the right lobe. The average cyst diameter was 9.6
cms (range 5 -13 cms). All type I and II hydatid cysts referred
to our department during this period, were treated with
puncture, aspiration, injection and re-aspiration (PAIR)
technique and were not included in this study. At each follow
up a through clinical examination and abdominal ultrasonography was performed. All ultrasonographic
examinations were evaluated by two authors (SSB and SM).
The diameter and echo pattern of the cysts were documented.
In addition, the liver, biliary tree, and other abdominal organs
were carefully examined for evidence of residual or recurrent
cysts.


Technique
Written informed consent was obtained and detailed technique,
other available modes of treatment, and complication rates
were explained to all the patients. The patients were subjected
to USG examination before the procedure. All patients received
oral albendazole 10mg /kg body weight, starting four week
pre-procedure and continuing for 4 weeks after the procedure.
After overnight fast an IV line was secured and 100 mg of hydrocortisone and 50 mg of diphenhydramine were given 20
minutes before the procedure. The procedure was performed
in the intervention suite under combined fluoroscopy and
ultrasound guidance, under local anesthesia and moderate
sedation. An anesthesiologist was always available to manage
potential anaphylaxis.
The cyst was punctured under sonographic guidance with
an 18 G needle. Longest available tract between the liver capsule
and the cyst wall was chosen as the site of puncture. The cyst
aspirate where obtained (n=7) was sent for microbiological
analysis for the presence of scolex and its mobility. A 0.035
super stiff guide wire (Cook, Bloomington Inc, USA) was
inserted into the cyst and the tract was sequentially dilated to
18 - 24 French. A large bore sheath (Amplatz Renal introducer
set, Cook Bloomington Inc, USA) was subsequently inserted
and stabilized manually. A suction catheter of 14 Fr size was
passed through it and the cyst contents were actively sucked
mechanically, using a suction apparatus. The catheter was
directed in different quadrants of cysts for effective removal.
Complete evacuation of solid portions (pearly white grapelike
membranes) (Figure 3) was attempted at the initial stage in all
patients. After satisfactory removal, the suction catheter was
withdrawn, retaining the large bore catheter. A cystogram was
then performed to look for any residual solid elements and to
demonstrate any biliary ductal communication. If more solid
elements were seen (Figure 4a), the suction procedure was
repeated until the clearance of cavity (Figure 4b). After
achieving cyst unilocularity, the large bore sheath was
exchanged with a 16 Fr to 20 Fr large bore catheter, which was
connected to a bag for drainage. The 48 hour drain output from
these catheters was sent for estimation of bile salts to reconfirm
lack of any possibility of biliary ductal communication.
Absolute alcohol, used as a scolicidal agent, was then instilled
in the cyst cavity, which was re-aspirated after 20 minutes.
Volume of absolute alcohol to be injected was taken as 50% of
the drain output in last 24 hours. The patients were discharged
48 hours later, after downsizing the catheter to 12-14 Fr. In one
patient who had 3 hydatid cysts, a two stage procedure was
performed, where two cysts were addressed in first, and the
third in the second session. No scolicidal/ sclerosing agent
was instilled in three patients who demonstrated biliary ductal
communication on repeat cystogram.


Follow up serial ultrasound and drainage pattern of cysts
were evaluated on outpatient visits every 7-10 days. When the
cyst aspirate decreased to <10 ml per 24 hours the catheter was
removed. In cases, where the cyst drainage fluid was bile stained
or the catheter output was persistently higher, the catheter
was retained for longer periods till spontaneous decrease in
output was encountered. In one patient there was persistent
output, even after 32 days, in whom endoscopic retrograde
cholangiography (ERCP) was performed and a plastic stent
was placed.
After catheter removal, follow-up sonogram was performed
at 1, 3, 6 and 12 months and every year thereafter. Decrease in
cyst size, progressive solidification, formation of a
pseudotumour or complete disappearance were regarded as
positive signs of response.
Results
The results are summarized in Table 2. The cysts were
completely evacuated in all patients. All patients tolerated the
procedure well, with no anaphylaxis. On microscopy, cyst
aspirate (obtained in n=7 patients) showed presence of
laminated membranes and protoscolices confirming that these
cysts were active. Cystogram immediately after the procedure
did not show any biliary ductal communication. However in 3
patients bilio-cystic communication was noted 48 hours later.
Spontaneous reduction in drain output was noted in 10 patients.
In 1 patient when the output did not reduce after 32 days,
ERCP with papillotomy and stenting was performed.
Subsequently this patient also showed diminished drain output.
Mean duration of catheter placement was 11.3 days (range 7 to
40 days). In uncomplicated patients (n = 8) the average duration
of hospital stay was 3 days (range 1 to 4 days). In patients with
bilio-cystic communication (n = 3) mean hospital stay was 4
days (range 3 to 6 days). Minor complications were encountered
in 7 patients in the form of urticaria (n = 3), reactive pleural
effusions (n = 3), pain (n = 6), and fever (n = 2). All patients
responded to symptomatic treatment. Follow up serial
ultrasound was performed on all patients. After percutaneous
treatment, the cysts gradually underwent a sequence of
sonographic changes from appearance of high-level internal
echoes (a heterogeneous pattern), obliteration by echogenic
material (a pseudotumor pattern), decreased echogenicity, and
finally a normal pattern of echoes. The cyst was considered to
have disappeared if it was no longer visualized on
ultrasonography and the area was replaced by an ill-defined
echogenic area or a normal echo pattern. Complete healing
was seen in 9 patients and formation of so called pseudotumour
in 4 patients. The mean follow up was 15.23 months (6 months
– 2 years).
Discussion
Percutaneous treatment of hepatic hydatidosis, initially
introduced in the mid-1980s, has developed into an attractive
alternative to surgery and benzimidazole derivatives for certain
cyst stages.[3,9] This treatment modality for hepatic hydatidosis
destroys the germinal layer with scolicidal agents or evacuates
the entire endocyst.[6] It has been tried with considerable
success, especially for the unilocular hepatic hydatid cysts,
with encouraging long term results.8,10,11,12,13,14 The first successful
percutaneous drainage of hepatic hydatid cyst was reported
by Mueller el al in 1985.[9] They drained the cyst percutaneously
under imaging guidance and then lavaged the cavity through
a catheter using silver nitrate and hypertonic saline solutions.
All liver function tests remained normal on one year follow up.
Subsequently in 1990, a new therapeutic approach involved
the following steps: puncture the cyst, aspirate cyst fluid, inject
a scolicidal agent, and re-aspirate the cyst content (PAIR).[10,11] Giorgio et al and Kabaalioglu et al reported repeated failures of
PAIR in multivesiculated cysts or cysts with predominantly
solid contents and daughter cysts, Gharbi type III (CE2 and
CE3B), possibly attributable to difficulty in extracting solid
components and cyst membranes.[15,16] Giorgio et al showed that
PAIR of multivesiculated cysts does not allow complete healing
and in 30% of cases resulted in an intracystic recurrence that
required up to four repeat procedures.[15,16] These findings
prompted most clinicians to use PAIR exclusively for unilocular
cysts, with or without detached endocysts. For this reason
Gharbi type III cysts are now preferably treated with other percutaneous techniques based on the aspiration of the “solid”
content of the cyst, the germinal and the laminated layer,
through a large-bore catheter or device.[6] Two types of
approaches are currently in use: the catheterization technique[17] and the modified catheterization techniques, in particular
PEVAC (percutaneous evacuation), MoCaT (modified
catheterization technique), and DMFT (dilatable multi-function
trocar).[6] Haddad et al used large-bore 14 Fr van Sonnenberg
sump catheters for drainage and suction of membranes in type
IV cysts.[18] We used larger 24 Fr bore catheter to achieve the
same. This along with wide distal end holes and val large side
holes offered better extraction of the solid cyst contents. We
found this to offer significant advantage for sufficient
evacuation of solid elements.
A large-bore cutting-aspiration device was used by Saremi
and McNamara in 32 patients to fragment and evacuate
daughter cysts and the laminated membrane.[19] A 2-year followup
showed a high rate of success (90%) and a low incidence of
major complications (3%). Vuitton et al treated 699
multivesiculated abdominal cysts with a device called DMFT
which was linked to an aspiration apparatus to extract the
endocyst, daughter cysts and other cystic contents.[20] Thereafter, the cavity was irrigated with 10–20% saline and if
necessary curettage was performed. The catheter remained in
the cavity for 2–3 days. No deaths but four anaphylactic
reactions were observed in their series. The recurrence rate in
a 3-year follow-up was 2.3% in-situ and 1% in other locations. Schipper et al treated 12 patients with unilocular and
multivesiculated cysts, including complicated ones with
PEVAC.[21] Aspiration and evacuation of cyst content were
performed with a 14 Fr catheter. Cysto-biliary fistulas were
treated with an endoprosthesis introduced endoscopically into
the common bile duct. In a mean period of 17.9 months (range
4 – 30 months) seven cysts disappeared and five decreased in
size. The main complications of this procedure were caused by
cysto-biliary fistulas and secondary infections. These
complications prolonged hospitalization from 11.5 (range 8–14
days) to 72.3 days (range 28–128 days).
Giorgio et al in their study proved efficacy of double
percutaneous aspiration and ethanol injection for previously
untreated patients with viable univesicular and multivesicular
hydatid liver cysts not only in the short term, but also over
long term and showed that this technique has a lower cost,
shorter hospital stay and fewer recurrences.1 We also used
absolute alcohol as scolicidal agent in hepatic hydatid cysts
which did not have any biliary ductal communication.
As compared to the available literature, results of our study
were better with mean duration of catheter placement being
11.3 days (range 7 to 40 days). Number of days of hospitalization
was only 3 days in uncomplicated patients (n = 8). In patients
with bilio-cystic communication (n = 3) mean hospital stay was
4 days (range 3 to 6 days). Complete healing was seen in 9
patients and formation of so called pseudotumour in 4 patients, which was chosen as end point of our study, thus showing a
100% success rate with a mean follow up of 15.23 months and
no recurrence.
The major risks of percutaneous techniques are
anaphylactic shock, secondary echinococcosis caused by
spillage of cystic fluid and chemical cholangitis caused by
contact of the scolicidal agent with the biliary tree. It is imperative to have resuscitation measures in place, to choose
a safe approach to the cyst, to give peri-interventional
prophylaxis with benzimidazoles and to exclude
communications with the biliary tree before injection of any
scolicidal agent.[6]
To avoid potential spillage we chose the longest available
tract between the liver capsule and the cyst wall as the site of
puncture. Thus the liver parenchyma can have a sealing effect
against the inadvertent spillage during the procedure
(tamponade effect). We ensured that all safety measures were
in place by giving 100 mg of hydrocortisone and 50 mg of
diphenhydramine 20 minutes before the procedure. The
procedure was performed under local anaesthesia but an
anesthesiologist was always available during the procedure to
manage potential anaphylaxis. No serious complications,
anaphylaxis or deaths were encountered in our series. Minor complications such as urticaria, fever and reactive pleural
effusion were encountered in 7 patients who responded to
symptomatic treatment. However three patients showed a minor
complication of biliary fistula which was managed by retaining
catheter for longer periods of time in 2 patients until the
spontaneous output reduced to <10cc/ day and by endoscopic
retrograde cholangiography (ERCP) with plastic stent
placement in 1 patient.
Prophylactic albendazole therapy was used in our study
with two considerations. First, in animal studies it has been
reported that albendazole therapy offers protective effect
against pro-spillage.[22] Khuroo et al in a prospective randomized
controlled study have shown that prophylactic albendazole
therapy prior to percutaneous treatment has better results as
compared with percutaneous treatment or albendazole therapy
alone.[23]
Thus we conclude that percutaneous treatment is a safe
and effective alternative therapy in treating Gharbi type III
hepatic hydatid cysts for patients who refuse surgery and
require a shorter hospital stay.
References
- Giorgio A, Di Sarno A, de Stefano G, Liorre G, Farella N,
Scognamiglio U, et al. Sonography and clinical outcome of viable
hydatid liver cysts treated with double percutaneous aspiration and ethanol injection as first-line therapy: efficacy and long-term follow-up. Am J Roentgenol. 2009;193:W186–92.
- Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am. 1996;25:655–89.
- Khuroo MS, Wani NA, Javid G, Khan BA, Yattoo GN, Shah AH, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997;337:881–7.
- Turgut AT, Akhan O, Bhatt S, Dogra VS. Sonographic spectrum of hydatid disease. Ultrasound Q. 2008;24:17–29.
- Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459–63.
- Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E.
Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79:301–11.
- Lucey BC, Kuligowska E. Radiologic management of cysts in the abdomen and pelvis. AJR Am J Roentgenol. 2006;186:562–73.
- Polat KY, Balik AA, Oren D. Percutaneous drainage of hydatid cyst of the liver: long-term results. HPB (Oxford). 2002;4:163–6.
- Mueller PR, Dawson SL, Ferruci JT Jr, Nardi GL. Hepatic echinococcal cyst: successful percutaneous drainage. Radiology. 1985;155:627–8.
- Filice C, Pirola F, Brunetti E, Dughetti S, Strosselli M, Foglieni
CS. A new therapeutic approach for hydatid liver cysts. Aspiration and alcohol injection under sonographic guidance. Gastroenterology. 1990;98:1366–8.
- Gargouri M, Ben Amor N, Ben Chehida F, Hammou A, Gharbi
HA, Ben Cheikh M, et al. Percutaneous treatment of hydatid cysts (Echinococcus granulosus). Cardiovasc Intervent Radiol. 1990;13:169–73.
- Salama H, Farid Abdel-Wahab M, Strickland GT. Diagnosis and treatment of hepatic hydatid cysts with the aid of echo-guided percutaneous cyst puncture. Clin Infect Dis. 1995;21:1372–6.
- Ustunsoz B, Akhan O, Kamiloglu MA, Somuncu I, Ugurel MS, Cetiner S. Percutaneous treatment of hydatid cysts of the liver: long-term results. Am J Roentgenol. 1999;172:91–6.
- Men S, Hekimoglu B, Yucesoy C, Arda IS, Baran I. Percutaneous treatment of hepatic hydatid cysts: an alternative to surgery. Am J Roentgenol. 1999;172:83–9.
- Giorgio A, Tarantino L, de Stefano G, Francica G, Mariniello N,
Farella N, et al. Hydatid liver cyst: an 11-year experience of treatment with percutaneous aspiration and ethanol injection. J Ultrasound Med. 2001;20:729–38.
- Kabaalioglu A, Ceken K, Alimoglu E, Apaydin A. Percutaneous imaging-guided treatment of hydatid liver cysts: do long-term results make it a first choice? Eur J Radiol. 2006;59:65–73.
- Akhan O, Dincer A, Gokoz A, Sayek I, Havlioglu S, Abbasoglu
O, et al. Percutaneous treatment of abdominal hydatid cysts with hypertonic saline and alcohol. An experimental study in sheep. Invest Radiol. 1993;28:121–7.
- Haddad MC, Sammak BM, Al-Karawi M. Percutaneous treatment of heterogenous predominantly solid echopattern echinococcal cysts of the liver. Cardiovasc Intervent Radiol. 2000;23:121–5.
- Saremi F, McNamara TO. Hydatid cysts of the liver: long-term
results of percutaneous treatment using a cutting instrument.
AJR Am J Roentgenol. 1995;165:1163–7.
- Vuitton DA, Zhi Wang X, Li Feng S, Sheng Chen J, Shou Li Y, Li
SF, et al. PAIR-derived US-guided techniques for the treatment
of cystic echinococcosis: a Chinese experience (e-letter).
Gut. 2002.
- Schipper HG, Lameris JS, van Delden OM, Rauws EA, Kager
PA. Percutaneous evacuation (PEVAC) of multivesicular
echinococcal cysts with or without cystobiliary fistulas which
contain non-drainable material: first results of a modified PAIR
method. Gut. 2002;50:718–23.
- Morris DL, Chinnery JB, Hardcastle JD. Can albendazole reduce the risk of implantation of spilled protoscoleces? An animal study. Trans R Soc Trop Med Hyg. 1986;80:481–4.
- Khuroo MS, Dar MY, Yattoo GN, Zargar SA, Javaid G, Khan
BA, et al. Percutaneous drainage versus albendazole therapy in hepatic hydatidosis: a prospective, randomized study. Gastroenterology. 1993;104:1452–9.