Jyoti R. Kini, Hema Kini, Muktha R. Pai, Sandeep Gopal Krishna N. R., B. V. Tantry
Associate Professor,
Department of Pathology,
Kasturba Medical College,
Mangalore, Karnataka, India.575001.
Corresponding Author:
Dr Jyoti R Kini
Email: kinijyoti@gmail.com
48uep6bbphidvals|314 48uep6bbph|2000F98CTab_Articles|Fulltext Primary neoplasms of the small intestine are extremely rare even though the small bowel constitutes 90% of the mucosal surface area of the gastrointestinal tract. They account for about 5% of all alimentary tract tumours.[1,2] Herein, we present two cases, one of Brunner’s gland hamartoma and the other Brunner’s gland hyperplasia.
Case Report
Case 1
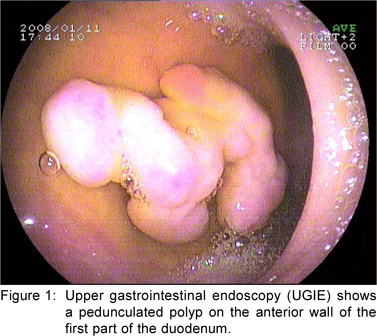
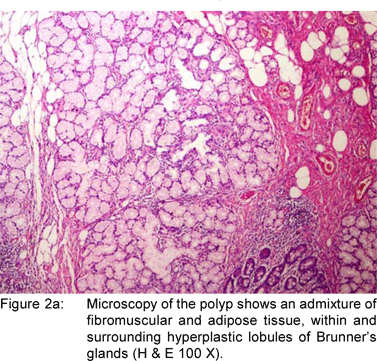
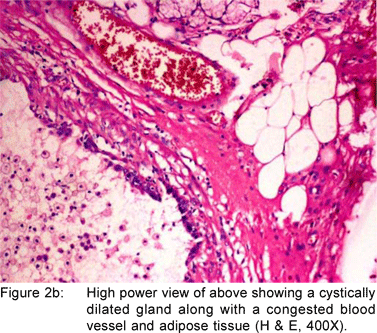
A 49 year old female presented with history of asthenia, abdominal discomfort and intermittent cough and fever of three months duration. She had undergone a computed tomography scan that revealed a mass lesion in the right lobe and the caudate lobe of the liver. Her serum alpha fetoprotein and carcinoembryonic antigen (CEA) levels were within normal limits. An upper gastrointestinal endoscopy (UGIE) revealed a three centimeter pedunculated polyp on the anterior wall of the first part of the duodenum (Figure 1). An endoscopic polypectomy was done and the polyp was submitted for histopathological evaluation. Microscopic examination revealed a polyp lined by normal duodenal mucosa with underlying subepithelial tissue containing an admixture of fibromuscular and adipose tissue, within and surrounding hyperplastic lobules of Brunner’s glands (Figure 2a). Some of the glands were cystically dilated (Figure 2b). Ducts, lymphoid tissue and numerous blood vessels were also present. A diagnosis of Brunner’s gland hamartoma was made. Liver biopsy showed features suggestive of a metastatic adenocarcinoma. She also had multiple peritoneal nodules in close proximity to the liver lesion. She refused further investigations and was discharged. Two months later, she returned with right sided pleural effusion. Cytology showed metastatic adenocarcinoma. The patient was discharged against medical advice and lost to follow-up.
Case 2
A 35 year old male was admitted with history of abdominal pain and dyspepsia of two months duration. UGIE revealed a polypoidal mucosal lesion at D1-D2 junction. Endoscopic biopsy of the lesion showed fragments of duodenal mucosa with intact surface epithelium, blunting of a few villi and numerous lobules of Brunner’s glands occupying more than 50% of the lamina propria. The rest of the lamina propria showed inflammatory infiltrate composed of lymphocytes, plasma cells, eosinophils and few neutrophils. Hyperplastic Brunner’s gland acini were also seen amidst the splayed muscularis mucosa. A diagnosis of Brunner ’s gland hyperplasia with active duodenitis was made.
Discussion
Brunner’s glands are submucosal mucin secreting glands localized predominantly in the duodenal bulb and proximal duodenum, decreasing progressively in size and number in the distal portions. The disease spectrum of Brunner’s glands includes diffuse nodular hyperplasia, circumscribed nodular hyperplasia (most common type), and adenomatous hyperplasia. Hyperplasia refers to multiple lesions less than one centimeter and adenoma refers to a single lesion larger than one centimeter. Brunner’s gland adenoma, also known as Brunneroma or polypoid hamartoma is a rare, benign, proliferative lesion arising from the Brunner’s gland of the duodenum accounting for 10.6% of benign tumours of the duodenum.[3] The exact pathogenesis of these lesions remains elusive. Currently, they are considered as hamartomas since they demonstrate an unusual admixture of normal tissues such as Brunner’s glands, ducts, adipose and lymphoid tissues. Thus, most tumours of Brunner’s glands in the duodenum represent hyperplastic or hamartomatous alternations although they may exceptionally evolve towards a malignant transformation.[4,5] In a study by Sakurai et al[6], dysplastic changes were seen in 2.1% and invasive carcinoma in 0.3% of all Brunner’s gland hyperplasia. In 1835, Curveilher described Brunner’s gland hyperplasia in a patient with duodenal intussusception.[7] Since then less than 200 cases have been reported in the literature.[8] These lesions account for less than 1% of small bowel tumours. The majority of cases are asymptomatic or present with nonspecific vague symptoms such as abdominal pain or discomfort, nausea or bloating. In the symptomatic patients, the most common clinical presentations are gastrointestinal bleeding and obstruction. Brunner’s gland hyperplasia is often associated with chronic renal failure, chronic pancreatitis, duodenitis and peptic ulcer[9]. Most patients present in the middle age and there is equal sex distribution.
Brunner’s gland hamartomas are mostly located in the duodenal bulb [57%]. Rarely, they may be found in the second [27%] and third [5%] parts of the duodenum, the pyloric canal [5%], the fourth part of the duodenum, jejunum and proximal ileum.[10] Brunner’s gland hyperplasia is characterized by lobules of glands that are increased in both size and number, and are separated by thin fibrous septae. These lobules extend from the submucosa into the lamina propria and within the mucosa alone and constitute atleast 50% of the length of the biopsy specimen. Brunner’s gland hamartoma consists of an admixture of fibromuscular and adipose tissue within and surrounding cystically dilated hyperplastic lobules of Brunner’s glands.[11] Upper gastrointestinal endoscopy with biopsy is the diagnostic method of choice. The differential diagnoses include polypoid adenoma of the superficial mucosal glands, adenoma of the islet cells, aberrant pancreatic tissue, lipoma, angioma, leiomyoma, gastrointestinal stromal tumor and malignant neoplasms like carcinoid, adenocarcinoma, lymphoma, leiomyosarcoma and metastasis. When the tumor is small or pedunculated, endoscopic polypectomy is the treatment of choice. Open surgical excision is reserved for cases where snaring has failed or when the neoplasm is too large. Endoscopic orsurgical excision is uncomplicated, and the long-term outcome is favorable.
References
1. Zollinger RM Jr. Primary neoplasms of the small intestine. Am J Surg. 1986;151:654–8.
2. Levine JA, Burgart LJ, Batts KP, Wang KK. Brunner’s gland hamartomas: clinical presentation and pathological features of 27 Cases. Am J Gastroenterol. 1995;90:290–4.
3. Chattopadhyay P, Kundu AK, Bhattacharyya S, Bandyopadhyay A. Diffuse nodular hyperplasia of Brunner’s gland presenting as upper gastrointestinal hemorrhage. Singapore Med J. 2008;49:81–3.
4. Brookes MJ, Manjunatha S, Allen CA, Cox M. Malignant potential in a Brunner’s gland hamartoma. Postgrad Med J. 2003;79:416–7.
5. Faller G, Kirchner T. Low grade intraepithelial neoplasia of Brunner’s gland. Histopathology. 2005;47:118–9.
6. Sakurai T, Sakashita H, Honjo G, Kasyu I, Manabe T. Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia. Am J Surg Pathol. 2005;29:1442–8.
7. Rocco A, Borriello P, Compare D, Colibus PD, Pica L, Iacono A, et al. Large Brunner’s gland adenoma: case report and literature review. World J Gastroenterol. 2006;12:1966–8.
8. Peetz ME, Moseley HS. Brunner’s gland hyperplasia. Am Surg. 1989;55:474–7.
9. Gao YP, Zhu JS, Zheng WJ. Brunner’s gland adenoma of duodenum: A case report and literature review. World J Gastroenterol. 2004;10:2616–7.
10. Iusco D, Roncoroni L, Violi V, Donadei E, Sarli L. Brunner’s gland hamartoma: ‘over–treatment’ of a voluminous mass simulating a malignancy of the pancreatic-duodenal area. JOP. 2005;6:348–53.
11. Patel ND, Levy AD, Mehrotra AK, Sobin LH. Brunner’s gland hyperplasia and hamartoma: imaging features with clinicopathologic correlation. AJR. 2006;187:715–22.
|