Pallavi Nigam1, Upama Misra2, T.S. Negi1, Balraj Mittal3, Gourdas Choudhuri1
*Department of Gastroenterology,
Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Lucknow,1
*Department of Pharmacy, SITM,
Barabanki,2
* Department of Genetics,
Sanjay Gandhi Postgraduate Institute of Medical Sciences,
Lucknow.3
Corresponding Author:
Dr. Gourdas Choudhuri
Email: gc@sgpgi.ac.in
Abstract
Background: Mutations in p53 gene are found in a majority of human malignancies and usually occur in the exons 5, 6, 7 and 8. Mutated p53 protein is more stable and gets accumulated in the cells that induce the host to develop anti-p53 antibodies in sera of cancer patients.
Aim: This study is aimed to observe the frequency and nature of mutations in exons 5-8 of p53 gene and to evaluate its correlation with prevalence of serum p53 antibodies in Indian patients with gallbladder cancer (GBC).
Methods: Mutation studies were done in cancer tissues obtained from 62 patients with proven GBC (40 cytologically proven cases and 22 resected gallbladder cancer tissues) by polymerase chain reaction (PCR), restriction fragment length analysis (RFLP) and single strand conformation polymorphism (SSCP). Presence of serum p53 antibodies was determined using highly specific enzyme linked immunosorbent assay (ELISA) kit in 50 patients with GBC and 30 patients of cholelithiasis. Clinicopathologic characteristics of these patients were given attention.
Results: Antibodies to p53 protein was present in the serum in 34% (17/50) of GBC patients and in 3.3% (1/30) patients with cholelithiasis (p<0.018). RFLP failed to detect common mutations in the exons 5- 8 of the p53 gene in 62 samples. Using SSCP analysis we could detect frameshift mutation in p53 gene in 2 of 22 (9.1%) GBC cases. Mutated samples were sequenced and found to have insertion of adenine at codon 271 (GAG) in exon 8 region.
Conclusion: Our results show that 1/3rd of the north Indian patients with GBC have antibodies to p53 protein. The commonest identifiable alteration in the p53 gene was a frameshift mutation at codon 271.
|
48uep6bbphidcol4|ID 48uep6bbphidvals|305 48uep6bbph|2000F98CTab_Articles|Fulltext Gallbladder cancer is an uncommon but highly malignant tumor. Females are two to six times more affected than males and usually develop this cancer in their late 50s.[1,2] There is clear worldwide association between GBC & gallstones.[2] Ethnic background & geographical location are important factors in the incidence of GBC. According to recent data published by Indian council of Medical Research, the highest GBC incidence worldwide to the tune of 21.5/100,000 for women was reported from northern India.[3] Apart from northern India, GBC occurs commonly in Japan and South American countries. Incidence of GBC also varies within a country. In India, the incidence is very high in the northern parts as compared to the south.[4]
In the search for effective prevention, diagnosis and treatment of this cancer, genetic changes involved in the origin of GBC should be determined. Most studies have focused on mutations of oncogenes K-ras or tumor suppressor genes p53, FHIT, p14 and p16 INK-4. K-ras oncogene mutations have been reported in 39-59% of GBC patients[5,6,7] and FHIT gene abnormalities are nearly universal in GBC. Abnormalities inp53 gene were seen in 35-92% of GBC.[2,8]
The inactivation of p53 gene plays a pivotal role in human tumorigenesis.[9] Alterations of p53 gene in human carcinogenesis can be assessed by using both molecular analysis of p53 gene and its product, the p53 protein. Mutant p53 protein is more stable (half-life of several hours compared with 20 min for wild-typep53), gets accumulated in the nucleus of neoplastic cells[10] and triggers development of immune response leading to the production of p53 antibodies in the sera.[10] Crawford et al[37] first detected antibodies in the serum to p53 protein in breast cancer patients followed later by their detection in various types of cancers including colorectal, ovarian, gastric, pancreatic and biliary.[11]. These antibodies are not present in the serum of patients without cancer.[12]
There are only few studies on the role of p53 alterations in GBC from India.[13,14] We therefore examined both p53 gene mutations and circulating p53 antibodies in GBC patients from north India, where GBC occurs commonly.
Methods
Patients and samples
112 patients with clinically proven GBC formed the cohort from which patients were included in the study. Serum samples from 50 and tissue samples from 62 GBC patients were collected: fine needle aspirate cytology (FNAC) samples from 40 patients and surgically resected fresh tissues from 22 patients of GBC undergoing surgery. 30 serum samples were collected from patients with cholelithiasis. All the sera and resected cancerous tissue were stored at -80°C. All the tissues were histopathologically confirmed for malignancy and diagnosis of cholelithiasis was based on the findings of ultrasonography. Written informed consent was obtained from all patients and the study protocol was approved by Ethical Committee of Sanjay Gandhi Post Graduate Institute of Medical Sciences.
Information about the invasive tumor included histological type and grade of differentiation, level of infiltration, presence or absence of gallstone, liver invasion and lymph node involvement. The cancers were classified according to the criteria established by the World Health Organization for the histological typing of tumors of the gallbladder and extra hepatic bile ducts.[15]
p53 gene mutation detection
DNA Extraction
Cells were scrapped off carefully from the FNAC slide after removing the cover slips. Scrapped cells were then treated at least four times with xylene followed by washing with 70% alcohol each time. The DNA was isolated from these cells by using QIAMP DNA mini kit (QIAGEN) as per the manufacturer’s instructions. Eluted DNA was stored at -80°C for further use.
Genomic DNA was isolated using standard phenolchloroform protocol from all 22 samples of surgically removed GBC tissues.
Polymerase chain reaction-Restriction fragment length analysis
DNA was amplified by polymerase chain reaction (PCR) using DNA thermal cycler. As the final concentration of extracted DNA was low, nested PCR was used to amplify exons 5-8 of p53 gene. The whole segment from exon 5-8 (inclusive) was amplified initially and then subsequently used as the template for amplification of each individual exon i.e., exon 5, 6, 7 and 8.[16] Using previously reported primers (Table 1), amplification was performed in 25 ml reaction volume containing 10 mM of each dNTPs (dATP, dCTP, dGTP, dTTP) (Bangalore Genie), 10 pmoles of each oligonucleotide primer.[16,17,18] and 0.5 U Taq DNA polymerase (Invitrogen). The amplified products were visualized using ethidium bromide stained 2% agarose gel. Amplification of the normal allele by PCR created a cleavage site for the restriction enzymes in the wild type, whereas mutant codon did not create the restriction enzyme cleavage sites. The sequence conforming to wild genotype at codons 175 (exon 5), 213 (exon 6), 249 (exon 7) and 282 (exon 8) were digested with restriction enzymes HhaI, TaqI, HaeIII and MspI respectively. Mutated exons remain undigested as their restriction sites are lost, and appear as single band on the ethidium bromide stained 10% poly acrylamide gel.
Single strand conformation polymorphism (SSCP)
5µl of each amplified sample (exon5, 6, 7 and 8) was denatured by mixing the samples in 15µl of denaturing dye and heating it to 95ºC. Denatured samples were then loaded into 12% of polyacrylamide gel. After electrophoresis at low temperature of 4ºC for 16-20 hours, the gel was silver stained to detect the mobility shift.
Gene sequencing
Any mobility shift in the SSCP gel was confirmed by gene sequencing of the same sample. Sequencing was performed commercially. The results were analyzed using software BioEdit and then the data was compared with the previous reports elsewhere.
Serum p53 Antibodies Assay
p53 antibodies were detected in stored serum samples of patients with GBC and cholelithiasis by sandwich enzymelinked immunosorbent assay using a commercially available kit (Pharma Cell, France). The assay was done according to the manufacturer’s instructions, with the following specifications: 1ml of 100 times diluted (X100) serum was incubated in micro titer wells coated with recombinant wild type human p53 protein for 60 minutes at 25°C to detect specific p53 antibodies and with a control protein to detect nonspecific interactions. After 2-3 washings, anti-human IgG antibodies conjugated with peroxidase was added for 60 minutes at 37°C followed by substrate addition for 30 minutes in dark. The enzyme process was stopped by adding 2N HCL and absorbance was measured at 450 nm at 37°C. The presence and concentration of p53 antibodies in a sample was determined by specific signals of the sample. The cut off point in the detection of antibody level was taken as 1 U/mL that corresponded to the specific signal of the lowest range positive control.
Results
Of 112 GBC patients, 80 were females (M:F 1:2.5) with mean age of 54.7 years; pain and jaundice were the most common presenting features in 91/112 (81%) and 58/112 (52%) respectively. Pruritus was present in 45 (40%) and cholangitis in 21 (19%) of them. Gallstones were present in 62/112 (55%) patients with GBC. The large majority of patients (96%) had advanced (stage III and IV) disease at the time of diagnosis. Only 5 patients (4%) had resectable (stage II) disease at the time of diagnosis. Most common area of contiguous spread in GBC patients was liver (23 patients). Lymph node, common bile duct, duodenum and brain metastasis was also found in various patients.
p53 gene mutation analysis
RFLP analysis of all the 62 GBC tissues corresponded to the wild genotype. No mutation was found in the known hot spot regions of codons 175, 213, 249 and 282 lying within exon5-8.
DNA from 22 resected GBC tissues was included in the SSCP analysis of exon 5-8 of p53 gene. PCR products obtained from malignant cells of FNAC slides were insufficient for running the SSCP experiment; hence FNAC slides were not included in the SSCP analysis of p53 gene.
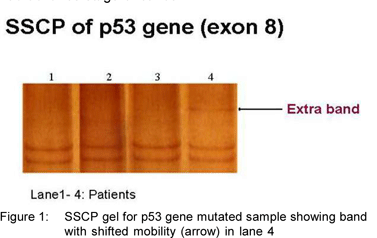
Two of the 22 tumors (9.1%) exhibited extra bands in the SSCP analysis of exon 8 (Figure 1: SSCP gel; Lane 4-mutated sample showing band shift), indicating possible mutations. Direct sequencing (Delhi University, New Delhi) revealed the insertion of adenine base at codon 271(GAG) leading to frame-shift mutation (Figure 2: Electropherogram of exon 8; sequence analysis showing adenine insertion at codon 271). Although there have been a few previous studies on the p53 gene mutations in GBC, the frame-shift mutation in codon 271 has not been reported, to our knowledge. Both the patients exhibiting mutations in codon 271 were female and had advance stage of cancer.
Serum p53 antibodies
p53 antibodies in the sera were detected in 17 (34%) of 50 patients with gallbladder cancer and 1 (3.3%) of 30 patients of cholelithiasis. Positive levels varied up to 2.7U/mL. Presence of p53-antibodies in GBC patients was significantly more than in controls (p<0.018).
The single patient with cholelithiasis, showing positive serum p53 antibodies, was found to have xanthogranulomatous cholecystitis on histopathological examination of resected gallbladder tissue.
Discussion
In the current investigation we evaluated the p53 gene alterations at gene and protein levels in patients of GBC in north Indian population and found that mutations are present in 9.1% (2/22) whereas p53 antibodies developed in the serum of 34% of the GBC patients.
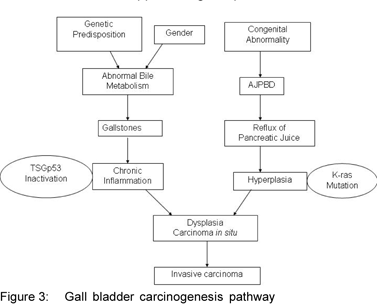
The rapid progress of molecular pathological research in recent years has revealed that various oncogenes and tumor suppressor genes are involved in stages of carcinogenesis, from initiation to growth and progression. Two main pathways of gallbladder carcinogenesis have been identified (Figure 3: GBC pathway). The most common is associated with gallstones and chronic inflammation of the gallbladder, whereas a second less frequent pathway is associated with congenital anomaly of the pancreatic bile-duct junction(AJPBD).[19] This AJPBD pathway has been reported mainly from Japan and has been reported to be frequently associated with mutations of the K-ras oncogene.[20] On the other hand, in GBC not associated with AJPBD, as is usually reported from other parts of the world, mutations are often found in the tumor suppressor gene p53.[21]
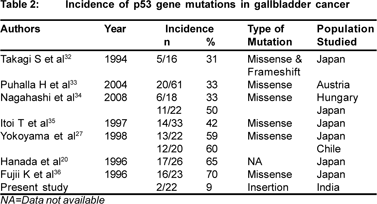
The reported frequencies of p53 gene mutations vary quite considerably for GBC from 35% to 92%.[2,8] while the present study showed the presence of low p53 gene mutations (9.1%) in GBC patients of north India. It is unlikely that just examining exons 5-8 missed any mutations, as >90% of the mutations documented in a wide range of tumors are found within this region. This discrepancy is attributed to several factors including methodologies.
The high frequency of p53 antibodies in the serum and much lower mutation frequency of p53 gene in the present study suggests that mechanisms other than p53 gene alterations stabilize the wild type p53 gene product. p53 gene can be inactivated by number of ways including p53 gene mutation, the most common mechanism, or defects in the cell pathways that regulate p53 levels or inhibit p53 function.[9] For example, the product of the MDM2 gene is known to bind to p53 protein and inhibit its ability to activate transcription. In some tumors, amplification of the MDM2 gene plays an important role in tumorigenesis, causing loss of the normal function of the p53 gene. Another reason for the discordance in the frequency of p53 antibodies and gene mutation can be the concept of mutations lying outside the region of exon5-8, which was not included in the present study.
An interesting observation has been the low rate of mutations in p53 gene reported from Indian subcontinent in cancers in general. The frequency has been 14% in hepatocellular carcinoma,[18] 3.3% in breast cancers,[22] 13.6% in glioma,[23] 10% in osteosarcomas[24] and 25% in oral cancers[25] in reports from India; these figures are much less compared with those from other parts of the world. This difference in frequency could be due to regional and ethnic differences, habitat and environmental factors that could be influencing carcinogenic pathways.
Mutation spectrum in p53 gene differs among various cancers with respect to the position and frequency of transition and transversion. p53 gene mutation occurs in evolutionary conserved codons of the p53 gene, which lies within exon 5- 8. Mutations lying outside these exons are rare.[26] Frequency of mutations in these exons in GBC has been reported in 50- 70% of patients from Japan and Chile.[27,28] In the present study,PCR-SSCP analysis of 22 gallbladder cancer patients, revealed p53 gene mutations in 2 (9.1%) of the patients at codon 271 (exon8). Also, no mutations were detected in any of the so called “hot spots” (codon 175, 213, 249 and 282) present between exon 5-8 by PCR-RFLP technique. Most of the reported p53 gene mutations that are associated with gallbladder cancer are of missense type (Table 2). In the present study, the characteristic of p53 gene mutation is frameshift mutation which results in disrupting the reading frame and finally a wrong protein, which will most likely be non-functional. This discrepancy in the type of mutations in our studies and previous other studies suggests the presence of several carcinogenic pathways of gallbladder cancer.
In carcinogenesis, mutations of the p53 gene leads to production of abnormal mutated protein, that is more stable and tends to accumulate in the cancerous cell nuclei. This accumulated protein is detected by immunostaining of the tissue containing cancerous cells. Over-expression of p53 protein in nuclei is found in wide variety of cancers including GBC.[10,11,12] In fact, 2 recent reports on GBC patients from northern India found over expression of p53 protein in 20 to 70% of cases.[13,14] by immunostaining of cancer tissues.
Antibodies are produced in response to the accumulation of p53 protein in the nucleus and can be detected in serum. p53 antibodies, first described 20 years ago, are found predominantly in human cancer patients with 96% specificity.[10] Presence of p53 antibodies has been commonly found in various cancers including gastric cancer,[12] cholangicarcinoma,[11] breast cancer, [29] colorectal cancer,[30] and esophageal cancer.[31] Our finding of the presence of this antibody in 34% of GBC patients in northern India is the first of its kind, and supports the previous findings of stable mutated p53 protein[13,14] in GBC.
However, our findings are based on a relatively limited number of cases, and a large number of samples are needed to better evaluate the role of p53 alterations in progression of GBC and as a prognostic factor.
To summarize, this investigation has demonstrated that p53 gene mutations are present in 9.1% (2/22) of the cases at codon 271 while 34% of GBC patients from north India are sero-positive for p53 antibodies. These results suggest the involvement of p53 gene mutations in the carcinogenesis of gallbladder in India.
References
1. Tada M, Yokosuka O, Omata M, Ohto, M, Isono K. Analysis of ras gene mutations in biliary and pancreatic tumors by polymerase chain reaction and direct sequencing. Cancer. 1990;66:930–5.
2. Lazcano-Ponce EC, Miquel JF, Munoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–64.
3. Nandakumar A, Gupta PC, Gangadharan P, Visweswara RN, Parkin DM. Geographic pathology revisited: development of an atlas of cancer in India. Int J Cancer. 2005;116:740–54.
4. Indian Council of Medical Research (ICMR). Annual report of population based cancer registries of the National Cancer Registry Programme (1993). New Delhi: ICMR Publication, 1996.
5. Wistuba II, Sugio K, Hung J, Kishimoto Y, Virmani AK, Roa I, et al. Allele-specific mutations involved in the pathogenesis of endemic gallbladder carcinoma in Chile. Cancer Res. 1995;55:2511–15.
6. Ajiki T, Fujimori T, Onoyama H, Yamamoto M, Kitazawa S, Maeda S, et al. K-ras gene mutation in gall bladder carcinomas and dysplasia. Gut. 1996;38:426–9.
7. Wistuba II, Ashfaq R, Maitra A, Alvarez H, Riquelme E, Gazdar AF. Fragile histidine triad gene abnormalities in the pathogenesis of gallbladder carcinoma. Am J Pathol. 2002;160:2073–9.
8. Misra S, Chaturvedi A, Goel MM, Mehrotra R, Sharma ID, Srivastava AN. Overexpression of p53 protein in gall bladder carcinoma in North India. Eur J Surg Oncol. 2000;26:164–7.
9. Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604.
10. Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–88.
11. Limpaiboon T, Sripa B, Wongkham S, Bhudisawasdi V, Chau-in S, Teerajetgul Y. Anti-p53 antibodies and p53 protein expression in cholangiocarcinoma. Hepatogastroenterology. 2004;51:25–8.
12. Maehara Y, Kakeji Y, Watanabe A, Baba H, Kusumoto H, Kohnoe S, et al. Clinical implications of serum anti-p53 antibodies for patients with gastric carcinoma. Cancer. 1999;85:302–8.
13. Misra S, Chaturvedi A, Goel MM, Mehrotra R, Sharma ID, Srivastava AN, et al. Overexpression of p53 protein in gallbladder carcinoma in North India. Eur J Surg Oncol. 2000;26:164–7.
14. Chaube A, Tewari M, Garbyal RS, Singh U, Shukla HS. Preliminary study of p53 and c-erbB-2 expression in gallbladder cancer in Indian patients. BMC Cancer. 2006;6:126.
15. Albores-Saavedra J, Henson DE, Sobin LH. The WHO Histological Classification of Tumors of the Gallbladder and Extrahepatic Bile Ducts. A commentary on the second edition. Cancer. 1992;70:410–4.
16. Doak SH , Jenkins GJ, Parry EM, Griffiths AP, Shah V, Baxter JN, et al. Characterisation of p53 status at the gene, chromosomal and protein levels in oesophageal adenocarcinoma. Br J Cancer. 2003;89:1729–35.
17. Coggi G, Bosari S, Roncalli M, Graziani D, Bossi P, Viale G, et al. p53 protein accumulation and p53 gene mutation in esophageal carcinoma. A molecular and immunohistochemical study with clinicopathologic correlations. Cancer. 1997;79:425–32.
18. Katiyar S, Dash BC, Thakur V, Guptan RC, Sarin SK, Das BC. P53 tumor suppressor gene mutations in hepatocellular carcinoma patients in India. Cancer. 2000;88:1565–73.
19. Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695–706.
20. Hanada K, Itoh M, Fujii K, Tsuchida K, Ooishi H, Kajiyama G. K-ras and p53 mutations in stage I gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Cancer. 1996;77:452–8.
21. Hanada K, Itoh M, Fujii K, Tsuchida A, Hirata M, Iwao T, et al. TP53 mutations in stage I gallbladder carcinoma with special attention to growth patterns. Eur J Cancer. 1997;33:1136–40.
22. Hedau S, Jain N, Husain SA, Mandal AK, Ray G, Shahid M, et al. Novel germline mutations in breast cancer susceptibility genes BRCA1, BRCA2 and p53 gene in breast cancer patients from India. Breast Cancer Res Treat. 2004;88:177–86.
23. Phatak P, Selvi SK, Divya T, Hegde AS, Hegde S, Somasundaram K. Alterations in tumour suppressor gene p53 in human gliomas from Indian patients. J Biosci. 2002;27:673–8.
24. Ramesh N, Vuayaraghavan AS, Desai BS, Natarajan M, Murthy PB, Pillai KS. Low levels of p53 mutations in Indian patients with osteosarcoma and the correlation with fluoride levels in bone. J Environ Pathol Toxicol Oncol. 2001;20:237–43.
25. Kannan S, Yokozaki H, Jayasree K, Sebastian P, Mathews A, Abraham EK, et al. Infrequent loss of heterozygosity of the major tumor suppressor genes in Indian oral cancers. Int J Oral Maxillofac Surg. 2002;31:414–8.
26. Hollstien M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53.
27. Yokoyama N, Hitomi J, Watanabe H, Ajioka Y, Pruyas M, Serra I, et al. Mutations of p53 in gallbladder carcinomas in high-incidence areas of Japan and Chile. Cancer Epidemiol Biomarkers Prev. 1998;7:297–301.
28. Roa I, Melo A, Roa J, Araya J, Villaseca M, de Aretxabala X. P53 gene mutation in gallbladder cancer. Rev Med Chil. 2000;128:251–8.
29. Mudenda B, Green JA, Green B, Jenkins JR, Robertson L, Tarunina M, et al. The relationship between serum p53 autoantibodies and characteristics of human breast cancer. Br J Cancer. 1994;69:1115–9.
30. Hammel P, Boissier B, Chaumette MT, Piedbois P, Rotman N, Kouyoumdjian JC, et al. Detection and monitoring of serum p53 antibodies in patients with colorectal cancer. Gut. 1997;40:356–61.
31. Shimada H, Nabeya Y, Okazumi S, Matsubara H, Funami Y, Shiratori T, et al. Prognostic significance of serum p53 antibody in patients with esophageal squamous cell carcinoma. Surgery. 2002;132:41–7.
32. Takagi S, Naito E, Yamanouchi H, Ohtsuka H, Kominami R, Yamamoto M. Mutation of the p53 gene in gallbladder cancer. Tohoku J Exp Med. 1994;172:283–9.
33. Puhalla H, Kandioler D, Ludwig C, Filipits M, Wrba F, Laengle F, et al. p53 analysis in gallbladder cancer: comparison of gene analysis versus immunohistochemistry. Anticancer Res. 2004;24:1201–6.
34. Nagahashi M, Ajioka Y, Lang I, Szentirmay Z, Kasler M, Nakadaira H, et al. Genetic changes of p53, K- ras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J Gastroenterol. 2008;14:70–5.
35. Itoi T, Watanabe H, Yoshida, Ajioka Y, Nishikura K, Saito T. Correlation of p53 protein expression with gene mutation in gallbladder carcinomas. Pathol Int. 1997;47:525–30.
36. Fujii K,Yokozaki H,Yasui W, Kuniyasu H, Hirata M, Kajiyama G, et al. High frequency of p53 gene mutation in adenocarcinomas of the gallbladder. Cancer Epidemiol Biomarkers Prev. 1996;5:461–6.
37. Crawford LV, Prin DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in the sera from patients with breast cancer. Int J Cancer. 1983;30:403–8.
|