48uep6bbphidcol2|ID
48uep6bbphidvals|3047
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
The peribiliary region comprises of portal vein, hepatic artery, nerves and lymphatics at porta. Hepatic parenchyma lies in the vicinity of primary and secondary biliary confluence. Periampullary structures such as pancreatic head and second part of the duodenum are other important structures surrounding the distal bile duct. Due to the close locationof structures in a compact space and various possible tissuesof origin (such as vascular, lymphatic or neural structures), determining the organ of origin and reaching a definite diagnosis are challenging. Table 1 enumerates the various peribiliary lesions which are subsequently discussed in detail in this review.
Peribiliary Cysts
Peribiliary cysts are an incidental finding in patients with advanced liver disease.They have been found to be prevalent in cirrhotic livers and in fibrocystic diseases such as Autosomal Dominant Polycystic Kidney Disease and Autosomal Dominant Polycystic Liver disease.1 These develop due to the disturbed periportal blood flow and periportal inflammation in cirrhosis which leads to the obstruction of peribiliary glands, causing their cystic dilatation and by genetic factors in fibrocystic diseases.1 As a result, these cysts have no communication with the bile duct lumen and lie outside the duct walls.1 Diagnosis of these cysts may help in detecting an underlying chronic liver disease in previously undiagnosed patients.2 Also the progressive increase in number and size of these cysts is indicative of worsening of liver disease.1,2 Portal hypertension, portal vein thrombosis, history of neoplasms, liver transplantation and primary sclerosing cholangitis are other associated conditions.1
Patients are usually asymptomatic. The finding of multiple, small cystic lesions (size ranging from 0.2 to 2.5 cm) predominantly around the hepatic confluence or larger portal tracts, on either side of the portal vein, not in communication with the biliary tree associated with an underlying cirrhotic liver is highly suggestive of the condition.2 Misdiagnosis of peribiliary cysts as dilated intrahepatic biliary radiclescan be avoided by the finding of cystic dilatation on both sides of the portal veins in peribiliary cysts, whereas dilation of intrahepatic bile ducts usually appears on one side.3 On USG, peribiliary cysts may be seen as echogenic portal tracts in cases with minute cysts or as hilar multicystic mass.2 On CT, they appear as a tubular structure coursing parallel to the portal veins or as a string of cysts which resemble abnormal bile ducts. Drip infusion cholangiographic computed tomography imaging (DIC-CT) has been found to distinguish biliary dilatation from peribiliary cysts due to the enhancement of the bile ducts in the former.1 However, its role is limited in patients with hepatic dysfunction.2 On MRI, peribiliary cysts are seen as fluid signal intensity lesions, hypointense on T1WI, hyperintense on T2WI with no postcontrast enhancement, and appear as string of beads lesions along the hepatic hilum and / or bile ducts.4 MRI with hepatobiliary contrast agents help to demonstrate non-communication of these cysts with biliary tree lumen since there is no contrast excretion within these lesions, while biliary radicles demonstrate excreted contrast within their lumen.
Rarely, the lesions can be seen adjacent to the extrahepatic biliary ducts as well.2 They are usually multiple in number and solitary lesions are found in 10% patients.1
Obstructive jaundice and cholangitis are the most commonly reported complications. Approximately 2% of the patients may present with signs and symptoms of biliary obstruction due to the extrinsic compressive effect on biliary tree.2 Another possible complication is that these cysts may act as neoplastic precursors due to the changes in peribiliary gland epithelium which may eventually give rise to intraductal papillary neoplasm of the bile duct, as well as cholangiocarcinoma.1
These lesions can at times be confused with malignant strictures such as in cholangiocarcinoma, especially when there is biliary ductal dilatation.4 MRCP is the modality of choice which helps to reach correct diagnosis and can differentiate from other close mimics as well such as intraductal papillary mucinous neoplasms of the bile ducts, cystic metastasis, Caroli’s disease, abscess formation, and primary sclerosing cholangitis, thus avoiding therapeutic misadventures. Correct diagnosis of this condition is essential since misdiagnosis as other lesions such as malignancy can cause unnecessary denials or delays in patients with end-stage liver disease who are acceptable candidates of liver transplantation.1,2
Biliary hamartoma (Von Meyenberg Complex (VMC)
Biliary hamartomas are congenital malformations of the biliary duct, resulting from failed involution of embryonic bile duct remnants, characterised by cystic dilatation of the bile ducts surrounded by fibrous stroma.5 They are detected incidentally on imaging in majority of cases.6 Biliary hamartomas, also known as von Meyenberg Complexes,occur aseither single or multiple, small sized cysts (1-15 mm in size) scattered throughout the liver parenchyma, especially in the subcapsular region.7
On USG, they appear as hypoechoic, hyperechoic or mixed heterogenic echoic structures, depending on the number and size of the cysts and the degree of fibrosis and often show multiple comet tail sign.8 On CT, they are seen as irregularly marginated, hypoattenuating lesions with no contrast enhancement. These lesions appear hypointense on T1WI and hyperintense on T2WI, compared to the surrounding liver parenchyma (Figure 1).8 The characteristic imaging findings help in the diagnosis; otherwise, followup imaging or histological confirmation is necessary to distinguish them from liver metastases, microabscesses, diffuse primary hepatocellular carcinoma, biliary cysts and Caroli’s disease.8
Infections
Abscess
Liver abscesses result because of ascending cholangitis, hematogenous dissemination from a gastrointestinal infection via the portal vein, disseminated sepsis via the hepatic artery or direct spread from contiguous organs.9 Patients most commonly present with fever and right upper quadrant abdominal pain.
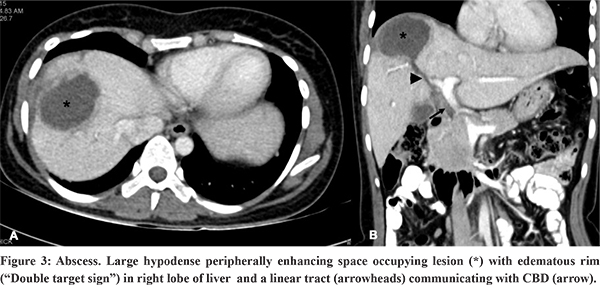
On USG, microabscessesare seen as either hypoechoic nodules or ill-defined areas of abnormal echotexture.9 Larger abscesses are usually hypoechoic space occupying lesions with variable echogenicity depending on the extent of liquefaction. Non-liquefied and early lesions may mimic a solid lesion. This dilemma can be solved by noting the posterior acoustic enhancement and absent internal colour flow on Doppler USG.9 On CT, abscesses appear as well-defined, hypodense, round or irregular shaped lesions with peripherally enhancing inner rim and hypoattenuating outer rim (“double target sign”)(Figure 2).9,10 Coalescing multiple small hypodense abscesses give rise to the “cluster sign”.9,10 These are associated with transient, early, wedge shaped adjacent parenchymal enhancement due to inflammation and compression of small portal venules due to mass effect resulting in reduced portal flow and a compensatory increase in hepatic arterial inflow. The presence of air allows a more confident diagnosis.5 On MRI, abscesses are centrally hypointense on T1WI, hyperintense on T2WI, but with variable internal signal intensity depending on the protein content. They often show an iso- to hypointense inner wall and a hyperintense outer wall (double target sign). The contents show restriction of diffusion on diffusion weighted MRI.9
USG guided aspiration or catheter drainage and antibiotic treatment is the usual line of management.9
Hydatid Cyst
Liver is the most commonly affected organ by hydatid cyst.11-13 Hydatid cysts are most commonly caused by either E.granulosus or E.multilocularis.11,13 Imaging findings correspond to the stage of cyst (ie, simple cyst with dependent debris, multilocular cyst containing daughter vesicles, partially or completely calcified cyst).11
One of the many complications of hydatid cyst is rupture into the biliary tree, or rarely the gallbladder.14 Rupture is more commonly seen with right lobe lesions (55-60% cases) than the left ones (25-30% cases).14 The rupture can be occult or frank, with frank rupture reported in 5-15% cases.14 Cystobiliary communication can be found in approximately 60% cases.12 Such patients present with jaundice, fever, and chills, due to biliary obstruction and cholangitis.
Cystobiliary communication is more likely to occur with large cysts (i.e., up to 80% of hepatic hydatid cysts larger than 7.5?cm), centrally located/ perihilar located cysts and in advanced stages of the disease.13 The compressive effect and resultant necrosis of the walls of adjoining biliary radicles due to the increased pressure within the cyst and incorporation of small biliary ducts within the pericyst, followed by the rupture of the biliary ducts are the possible mechanisms leading to cystobiliary communication.13
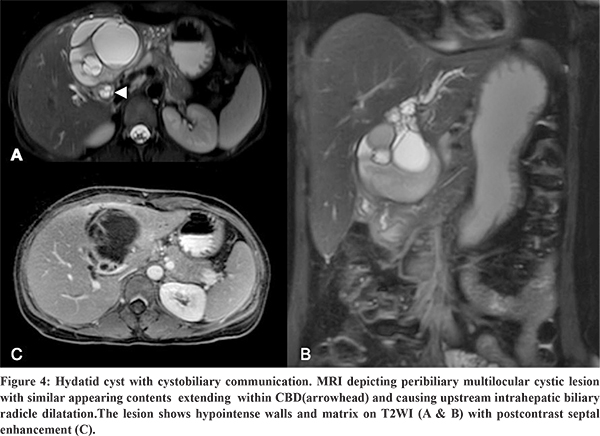
USG, CT, MRCP, ERCP are the diagnostic imaging modalities used in the evaluation of hydatid cysts. The typical radiological finding of a hydatid cyst ruptured into biliary tree is the disruption of the cyst wall with evidence of a connection between the bile ducts and the cyst with contents of the hydatid cyst within the biliary ducts.12 On USG, this finding is seen as anechoic cystic structures or echogenic linear membranes within the biliary tract with upstream biliary radicle dilatation.12 Corresponding imaging findings on CT are interrupted or irregular cyst wall adjacent to a bile duct, intrahepatic biliary radicle dilatation, linear membranes or high-density material within the bile ducts.12 Intra cystic fat-fluid level is an indirect sign of cystobiliary communication.13 MRI or MRCP findings suggesting an occult fistula include focal deformation or focal defect of the cyst wall or a beak-like projection extending from the cyst wall and intraductal contents (Figure 3) appearing hyperintense on T1WI and hypointense on T2WI.13 Direct communication of the hydatid cyst with biliary tract can be demonstrated using hepatobiliary contrast agent which shows excreted contrast within the cyst.13 Preoperative ERCP is more sensitive (86-100% sensitive) in the visualisation of the cysto-biliary communication.13 Presurgical clearance of the biliary tree and sphincterotomy is known to reduce the incidence of postoperative complications in patients with hepatic hydatid cyst with cysto-biliary communication.15
Inflammatory
Inflammatory pseudotumor of biliary tract (IPT)
IPT is a benign, inflammatory mass which closely resembles malignancy.16 These lesions present with diagnostic dilemma due to the non-specific clinical presentation, laboratory parameters and imaging features. Although, hepatic IPTs may be seen in any age groups,they are more common in children and younger adults.17 Majority ofthe patients present with fever, epigastric or abdominal pain, malaise and weight loss.17 Obstructive jaundice, hepatomegaly, splenomegaly and portal hypertension are other findings.17 Laboratory evaluation show elevated total leucocyte count, ESR and CRP, all of which are suggestive of an ongoing inflammatory process. Other findings include anemia, thrombocytosis, elevated IgG levels,elevated liver enzymes and lactate dehydrogenase.
Imaging findings are highly variable and non-specific.16,17 Masses can be very large with sizes upto 25 cm, which may cause obstructive jaundice. IPTs are usually ill-defined. These are hypoechoic on USG, have low attenuation on CT and appear hypointense on T1 and hyperintense on T2 weighted MRI.17 They typically demonstrate peripheral contrast enhancement with delayed central enhancement on CT and MRI, due to the fibrous composition of the tumor.17 This pattern of delayed enhancement is also seen in cholangiocarcinoma and metastasis. Histopathological confirmation through percutaneous biopsy can help reach proper diagnosis, and decide the line of management.16,17
Medical management with antibiotics or NSAIDs may lead to regression of the tumor in some cases within 2-6 months.17 IPTs may regress spontaneously as well.17 Surgical resection is often undertaken to exclude a malignancy or in patients who do not respond to medical management.17 However, radiologists must be aware of IPT recurrence and malignant transformation which may occur years following resection.
Biloma
Bilomas are extrabiliary collections of bile which can be intra- or extrahepatic, encapsulated or unencapsulated.18 They can result from trauma, iatrogenic causes (post transarterial chemoembolization (TACE), percutaneous ethanol injection, microwave ablation,15 or post cholecystectomy; post cholecystectomy is the most common iatrogenic cause) or may be spontaneous.18 Iatrogenic causes like TACE or arterial injury cause biloma as a result of ischaemic cholangiopathy. Diminished arterial blood flow to bile duct wall leads to bile duct wall necrosis, resulting in bile leakage, biloma formation and sepsis. Ischaemic cholangiopathy is also a complication of liver transplantation, radiation therapy and vasculitis.
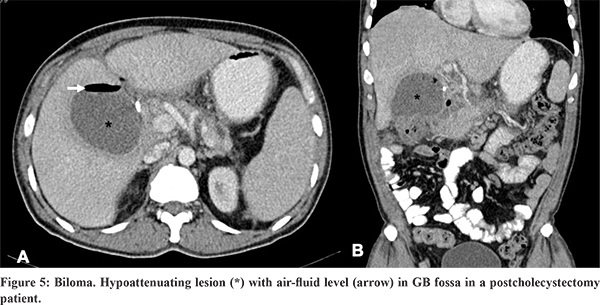
Seventy percent of bilomas are localized to the right upper quadrant (intrahepatic or located in the right subphrenic or subhepatic region), whereas the remaining 30% develop in the left upper quadrant.15 Ischaemic cholangiopathy results in intrahepatic or peribiliary collections. On USG, bilomas appear as hypoechoic collections with dependent debris, while on CT these appear as fluid attenuation collections.10 Bilomas have variable signal intensity on T1WI , and appear hyperintense on T2WI.18 A delayed phase MRI examination using hepatobiliary agents confirms bilious origin of a localized fluid collection and identifies the site of bile leak.15 It is also useful for confirmation of an active bile leak.18
Treatment options include imaging guided pigtail drainage or surgical drainage. Management of bilomas can also involve treating any associated biliary tract obstruction which can both complicate and cause bilomas. Surgical repair of the source of underlying biliary tract bile leak may also be required.
Neoplasms
Benign
Biliary Cystadenoma
Biliary cystadenoma is a rare, benign, cystic neoplasm arising predominantly in the intrahepatic biliary radicles, although it may be located in extrahepatic bile ducts and gall bladder.19,20 It is more commonly encountered in females of mean age around 45 years.19,20 The affected individuals usually have elevated bilirubin and transaminase levels.20 Increased levels of CA19-9 and CEA are also found.20
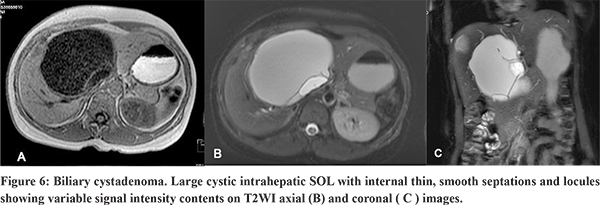
Radiological examination shows large cystic masses with sizes ranging from 3 to 40 cm, and can be unilocular or multilocular.19,21 On USG, they may appear to be completely anechoic or show low-level internal echoes may be seen as a result of blood products, mucin or proteinaceous fluid and septations.21 On CT, the masses are usually of fluid attenuation and may show septal or wall calcification. On MRI, the signal intensity is variable, depending upon the protein content or blood products.21 Commonly, it is hypointense on T1WI and less hyperintense than GB on T2WI. Presence of mural nodule , thick irregular wall, thick calcification and papillary projection should raise asuspicion of cystadenocarcinoma, the malignant counterpart of this entity.20,22 However, it is difficult to distinguish between these two entities confidently on imaging alone. Hence, it is recommended that all cystic lesions with possibility of biliary cystadenomas should be surgically excised.20
Intrahepatic Bile Duct Adenoma (BDA)
Intrahepatic bile duct adenoma is a rare, benign solid tumor arising from the bile duct epithelium.20 It is a small, well defined lesion of size usually less than 2 cm, in subcapsular location of liver.20 On MRI, the lesion has variable signal on T1 WI and appears hyperintense on T2 WI. On dynamic contrast study, the lesion shows enhancement during the arterial phase with persistence of enhancement during the portal and delayed phases due to inflammatory and fibrotic composition of the lesion.20
Malignant
Periductal Infiltrating Cholangiocarcinoma
Cholangiocarcinoma is one of the most frequently encountered biliary malignancies. This malignancy has three morphological forms- intraductal-polypoidal, periductal-infiltrating and intrahepatic mass forming.23 The periductal-infiltrating cholangiocarcinoma spreads along the periductal or perineural connective tissues and lymphatics even with intact mucosa, whereas the intraductal type spreads superficially along the mucosa.24 The periductal-infiltrating type shows a worse prognosis than the intraductal-growing type.23,24 On CT and MRI, periductal cholangiocarcinoma manifests as periductal asymmetrical, irregular soft tissue thickening along the narrowed bile ducts with dilatation of the proximal bile ducts (Figure 6). The soft tissue may encase the portal vein and hepatic artery.23,24 The periductal soft tissue thickening is usually segmental or focal rather than diffuse. This helps to differentiate from lymphatic dissemination of extrahepatic malignancies.23 On multi-phase CT, the lesion may show enhancement in the arterial phase and retains contrast in the portovenous and delayed phases owing to the scirrhosis nature of the tumor.23,24 Diffusion weighted imaging and multiphasic dynamic fat-saturated 3D gradient-echo T1-weighted imaging (T1WI) are the most useful sequences in evaluation of cholangiocarcinomas.
Peribiliary Metastasis
Metastasis in peribiliary location are rare; however, these are the most common peribiliary malignancies after cholangiocarcinomas. Gastrointestinal malignancies (such as colorectal, gastric, and pancreatic carcinoma) are the most common primaries which metastasise to the biliary tract.20 Malignancies of the breast, lung, and kidneys, melanoma and lymphoma are other malignancies which can spread to the biliary tract.20 Common hepatic duct is the most common involved site.20 The involvement is seen either as an extraluminal mural tumor tissue or as hepatoduodenal ligament lymph node metastasis.20
Radiological imaging aims to detect the lesion, characterise it and assess preoperative resectability. CEUS, CT and MRI when used collectively help in giving adequate information. MRI is considered a better modality than CT for detecting metastasis.20,25 Small lesions are very difficult to detect on CT because the lesion characteristic is difficult to differentiate from that of the adjacent liver parenchyma. Indirect signs such as bile duct dilatation are more helpful in detecting larger lesions, and this finding can direct the patient to an MRI for further evaluation. Peribiliary metastasis are best detected on T2W MRI and DWI.25 The high spatial and contrast resolution allow accurate tumor detection and staging. PET is a sensitive technique for the detection of peribiliary tumors, it is not specific in their differential diagnosis. Diagnosis established by histopathological study.25
Peribiliary Chloroma
Chloromas are localized extramedullary proliferation of the primitive malignant myeloid cells.23 These are more often seen in acute than in chronic myeloid leukemia. They most commonly manifest during there lapse or remission phase of the disease and mark poor disease prognosis. Leukemic infiltration is more common in bones, lymph nodes, soft tissues, skin and breast, while liver is a rare site.23
Peribiliary chloroma manifests as circumscribed or infiltrative lesions as a result of infiltration of hepatic sinusoids and peribiliary venous plexus by myeloid cells.23 These lesions are hypoattenuating with no or mild contrast enhancement. Chloromas may cause mass effect with resultant obstructive jaundice. Abscesses and lymphoma are close differentials of this condition. Obstructive jaundice is a poor prognostic factor due to complications of cholangitis and adverse effects of chemotherapy. Early detection of chloroma in initial stages can be treated with chemotherapy or focal ablative procedure.23
Lymphoma
B-cell lymphoma is the most common form of lymphoma, which can either be primary or secondary.20 Hepatic lymphomas have varying appearances. These can be seen as discrete focal lesions, diffuse infiltrating lesions, or an ill-defined masses in the porta hepatis. They can track through the peribiliary tissue without causing extrinsic compression of the bile ducts.20 The lesions can be solitary or multiple and appear hypoechoic on USG, hypoattenuating on CT with mild contrast enhancement. Calcification is a feature observed post chemotherapy. On MRI, the lesions are hypointense on T1WI , moderately hyperintense on T2WI, show diffusion restriction with progressive contrast enhancement.20 Primary lymphomatous involvement most commonly manifests as a solitary discrete lesion in approximately 60% cases. Multiple lesions are less common and a single dominant lesion is more commonly found in such cases.23 This is an important key feature of primary lymphoma as dominant lesions are usually not found in secondary lymphomas. Secondary hepatic lymphomas usually manifest as multifocal lesions or show diffuse infiltration in about 90% patients.23 Another feature which may distinguish primary from secondary lymphomas is the homogeneity of the lesion in primary lymphoma despite their large sizes, whereas heterogenous enhancement is observed in secondary lymphomas. The lesions show hypermetabolism on FDG PET/CT. PET/CT is usually the imaging modality of choice for staging and for assessing treatment response.
Vascular
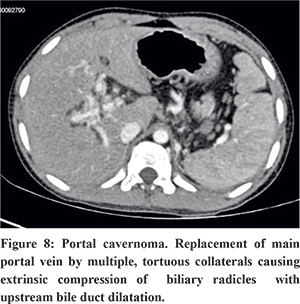
Portal Cavernoma
Portal cavernoma refers to replacement of main portal vein by multiple, tortuous collaterals as a result of portal vein thrombosis.26 Neonatal umbilical sepsis and dehydration are the underlying causes in children, whereas hypercoagulable states, myeloproliferative disorders, Bechet’s syndrome, pancreatitis and pylephlebitis are the common causes in adults. On USG, multiple tortuous vascular channels are seen at liver hilum. Multiphase CT (Figure 7) and MRI show enhancement of tortuous vascular channels in portovenous phase. These collateral channels can cause extrinsic compression of intra or extrahepatic biliary radicles with upstream bile duct dilatation referred to as portal biliopathy or portal cavernoma cholangiopathy.
Hepatic Artery Aneurysm and Pseudoaneurysm
Hepatic artery aneurysm is one of the most common types of abdominal visceral artery aneurysms. Extrahepatic arteries are involved in about 80% cases.27 Such aneurysms are more life threatening because of the lack of tamponade effect by surrounding hepatic parenchyma. Atherosclerosis is the most common underlying cause.27 Hepatic artery pseudoaneurysms (Figure 8) are usually the result of blunt or penetrating trauma, inflammatory conditions like pancreatitis or occur due to iatrogenic causes such as liver transplantation, indwelling biliary catheter or percutaneous and laparoscopic biliary procedures.27
Mostly hepatic artery aneurysms are found incidentally on imaging. Symptomatic patients with hepatic artery aneurysms or pseudoaneurysms may present with right upper quadrant pain, hemobilia, gastrointestinal hemorrhage, chronic anemia, and jaundice due to extrinsic compression of the bile ducts. A triad of epigastric pain, hemobilia, and obstructive jaundice (Quincke triad) is seen in up to one-third of cases.27 CT Angiography is the non-invasive imaging modality of choice for diagnosing aneurysms and pseudoaneurysms. One should search for the possibility of aneurysm multiplicity, which is known to occur in 20% of hepatic artery aneurysms.
Treatment of a hepatic artery aneurysm is indicated if the diameter exceeds 2 cm or if the patient is symptomatic. Special consideration is given to high-risk lesions (eg, in patients with polyarteritis nodosa or fibromuscular dysplasia, in whom treatment is recommended regardless of lesion size).27
Intrahepatic aneurysms and pseudoaneurysms are typically managed with endovascular embolization, whereas the management of involved extrahepatic arteries varies and the management options are open surgical repair, endovascular embolization, and endograft placement.27
Miscellaneous
Hepatic Plexiform Neurofibromas (NF)
Hepatic plexiform neurofibroma is rare and associated with NF-1 (Von Recklinghausen’s disease).28 It arises from the hepatic nerve plexus comprised by autonomic nerve branches of splanchnic and phrenic nerves, which course along the portal vessels and biliary tracts.28 These are usually seen in cases of generalised abdominal and retroperitoneal involvement.29
These seen as sheath-like, multilobulated, fusiform, homogenously hypodense lesions,with none to mild enhancement, and have diffuse or segmental distribution in periportal location.30 The hypoattenuating nature can be explained by the myxoid matrix, entrapped adipose tissue and cystic degeneration. On MRI, these appear as “target-like” lesions which show central hypointensity of collagenous or fibrotic component and peripheral hyperintensity of myxoid or cystic degeneration component on T2WI with central post gadolinium enhancement.30 NF characteristically encases the portal vessels and biliary radicles without exerting any mass effect on these structures. This periportal infiltration with normal vessel distribution is a helpful feature in distinguishing from other perihilar infiltrating lesions such as lymphomas, mesenchymal tumors, atypical cystadenocarcinomas, and mixed epithelial and mesenchymal tumors.29 Continuous extension of the lesion from the retroperitoneum and/or mesenteric root is another hallmark feature.23
The preferred management of these tumors is biopsy and surgical resection due to risk of possible malignant transformation.31 However, resection is often difficult as vessels and biliary ducts course through the mass. Hence, follow-up imaging is necessary. Rapid growth and heterogenous enhancement of the mass are pointers towards malignant transformation.31
Hepatic Langerhans Cell Histiocytosis (LCH)
Hepatic LCH is an often over looked entity,which is much more common as a part of disseminated disease(representing 40% to 60% of cases)and is associated with poor prognosis.32 The radiological features represent two stages of the natural history of LCH i.e. early infiltration by histiocytes and late sclerosis of the biliary tree.32 Hepatic LCH in children typically presents with hepatomegaly, deranged liver function tests and jaundice.33 Hepatomegaly is a manifestation of direct infiltration by Langerhans cells. Langerhans cells and other inflammatory cells infiltration lead to periportal proliferative and granulomatous lesions, which are seen as periportal hypoechoic bandlike or nodular areas on USG, hypoattenuating soft tissue on CT (Figure 9A), and intermediate to high signal intensity lesion on T2WI.32,34 Periportal contrast enhancement is suggestive of portal triaditis.32 Also hepatic parenchymal nodules with ring enhancement can be seen on CT (Figure 9B) which may mimic metastasis or granuloma. Periductal fibrosis and micronodular biliary cirrhosis resulting from sclerosing cholangitis characterise the fibrous stage of the disease.32 This fibrous stage of the disease may progress despite resolution of Langerhans cells.32
Patients with hepatic LCH are treated with chemotherapy.However,the disease may progress despite treatment.32,33 Knowledge of the imaging features of hepatic LCH is necessary since patient prognosis and treatment changes drastically in patients with liver involvement due to worse prognosis and increased mortality in patients with hepatic involvement.32
Conclusion
Peribiliary lesions may often pose a diagnostic challenge. Knowledge about the key features of various conditions (Table 2) can help solve the dilemma in many cases and guide proper treatment.
References
- Bazerbachi F, Haffar S, Sugihara T, Mounajjed TM, Takahashi N, Murad MH, et al. Peribiliary cysts: a systematic review and proposal of a classification framework. BMJ Open Gastroenterology. 2018 Jun 1;5(1):e000204.
- AlNuaimi D, Balci NC, AlDuaij A, AlKetbi R, Sierra MPR. A case of peribiliary hepatic cysts in a cirrhotic liver: A mimicker of Klatskin Tumor. Radiol Case Rep. 2020 May 15;15(7):1039–43.
- Ines DD, Essamet W, Montoriol PF. Peribiliary cysts. Hepatology. 2011;54(6):2272–3.
- Lim J, Nissen NN, McPhaul C, Annamalai A, Klein AS, Sundaram V. Peribiliary hepatic cysts presenting as hilar cholangiocarcinoma in a patient with end-stage liver disease. J Surg Case Rep [Internet]. 2016 Aug 9 [cited 2021 Feb 15];2016(8). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4979529/
- Jáquez-Quintana JO, Reyes-Cabello EA, Bosques-Padilla FJ. Multiple Biliary Hamartomas, The ‘“Von Meyenburg Complexes.”’ Ann Hepatol. 2017 Sep 1;16(5):812–3.
- Zheng R-Q, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol. 2005 Oct 28;11(40):6354–9.
- Chung EB. Multiple bile-duct hamartomas. Cancer. 1970 Aug;26(2):287–96.
- Barboi O, Moisii L, ALBU-SODA A, CIORTESCU I, Drug V. Biliary Hamartoma. Clujul Medical. 2013 Nov 6;86:383–4.
- Bächler P, Baladron MJ, Menias C, Beddings I, Loch R, Zalaquett E, et al. Multimodality Imaging of Liver Infections: Differential Diagnosis and Potential Pitfalls. RadioGraphics. 2016 May 27;36(4):1001–23.
- Malekzadeh S, Widmer L, Salahshour F, Egger B, Ronot M, Thoeny HC. Typical imaging finding of hepatic infections: a pictorial essay. Abdom Radiol. 2021 Feb 1;46(2):544–61.
- Mehta P, Prakash M, Khandelwal N. Radiological manifestations of hydatid disease and its complications. Trop Parasitol. 2016;6(2):103–12.
- Alan B, Kapan M, Teke M, Hattapoglu S, Arikanoglu Z. Value of cyst localization to predict cystobiliary communication in patients undergoing conservative surgery with hydatid cyst. Ther Clin Risk Manag. 2016 Jun 15;12:995–1001.
- Greco S, Cannella R, Giambelluca D, Pecoraro G, Battaglia E, Midiri M, et al. Complications of hepatic echinococcosis: multimodality imaging approach. Insights into Imaging. 2019 Dec 2;10(1):113.
- Wani I, Bhat Y, Khan N, Mir F, Nanda S, Shah OJ. Concomitant Rupture of Hydatid Cyst of Liver in Hepatic Duct and Gallbladder: Case Report. Gastroenterology Res. 2010 Aug;3(4):175–9.
- Dolay K, Akbulut S. Role of endoscopic retrograde cholangiopancreatography in the management of hepatic hydatid disease. World J Gastroenterol. 2014 Nov 7;20(41):15253–61.
- Tublin ME, Moser AJ, Marsh JW, Gamblin TC. Biliary Inflammatory Pseudotumor: Imaging Features in Seven Patients. American Journal of Roentgenology. 2007 Jan 1;188(1):W44–8.
- Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory Pseudotumor: The Great Mimicker. American Journal of Roentgenology. 2012 Mar 1;198(3):W217–27.
- Copelan A, Bahoura L, Tardy F, Kirsch M, Sokhandon F, Kapoor B. Etiology, Diagnosis, and Management of Bilomas: A Current Update. Techniques in Vascular and Interventional Radiology. 2015 Dec;18(4):236–43.
- Soares KC, Arnaoutakis DJ, Kamel I, Anders R, Adams RB, Bauer TW, et al. Cystic Neoplasms of the Liver: Biliary Cystadenoma and Cystadenocarcinoma. J Am Coll Surg. 2014 Jan;218(1):119–28.
- Granata V, Fusco R, Catalano O, Filice S, Avallone A, Piccirillo M, et al. Uncommon neoplasms of the biliary tract: radiological findings. BJR. 2017 Oct;90(1078):20160561.
- Soochan D, Keough V, Wanless I, Molinari M. Intra and extra-hepatic cystadenoma of the biliary duct. Review of literature and radiological and pathological characteristics of a very rare case. Case Reports. 2012 Apr 4;2012:bcr0120125497.
- Billington PD, Prescott RJ, Lapsia S. Diagnosis of a biliary cystadenoma demonstrating communication with the biliary system by MRI using a hepatocyte-specific contrast agent. Br J Radiol. 2012 Feb;85(1010):e035–6.
- Singh A, Chandrashekhara SH, Handa N, Baliyan V, Kumar P. “Periportal neoplasms”—a CT perspective: review article. Br J Radiol [Internet]. 2016 Apr [cited 2020 Dec 20];89(1060). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4846206/
- Lee DH, Kim B, Lee ES, Kim HJ, Min JH, Lee JM, et al. Radiologic Evaluation and Structured Reporting Form for Extrahepatic Bile Duct Cancer: 2019 Consensus Recommendations from the Korean Society of Abdominal Radiology. Korean Journal of Radiology. 2021 Jan 1;22(1):41–62.
- Granata V, Fusco R, Setola SV, Avallone A, Palaia R, Grassi R, et al. Radiological assessment of secondary biliary tree lesions: an update. J Int Med Res. 2020 Jun 1;48(6):0300060519850398.
- Kalra N, Shankar S, Khandelwal N. Imaging of Portal Cavernoma Cholangiopathy. J Clin Exp Hepatol. 2014 Feb;4(Suppl 1):S44–52.
- Jesinger RA, Thoreson AA, Lamba R. Abdominal and Pelvic Aneurysms and Pseudoaneurysms: Imaging Review with Clinical, Radiologic, and Treatment Correlation. RadioGraphics. 2013 May 1;33(3):E71–96.
- Garrouche N, Abdallah AB, Arifa N, Hasni I, Cheikh YB, Farhat WB, et al. Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights Imaging. 2018 Oct;9(5):661–71.
- Malagari K, Drakopoulos S, Brountzos E, Sissopulos A, Efthimidadou A, Hadjiyiannakis E, et al. Plexiform Neurofibroma of the Liver: Findings on MR Imaging, Angiography, and CT Portography. American Journal of Roentgenology. 2001 Feb;176(2):493–5.
- Khandwala K, Sajjad Z, Abbasi S, Tariq MU. Hepatic, Periportal, Retroperitoneal, and Mesenteric Neurofibromatosis in von Recklinghausen’s Disease. Cureus [Internet]. 2018 Feb 28 [cited 2021 Feb 22];10(2). Available from: https://www.cureus.com/articles/11126-hepatic-periportal-retroperitoneal-and-mesenteric-neurofibromatosis-in-von-recklinghausens-disease
- Ghalib R, Howard T, Lowell J, Huettner P, Whelan A, Teefey S, et al. Plexiform neurofibromatosis of the liver: Case report and review of the literature. Hepatology. 1995;22(4):1154–7.
- Yi X, Han T, Zai H, Long X, Wang X, Li W. Liver involvement of Langerhans’ cell histiocytosis in children. Int J Clin Exp Med. 2015 May 15;8(5):7098–106.
- Schmidt S, Eich G, Geoffray A, Hanquinet S, Waibel P, Wolf R, et al. Extraosseous Langerhans Cell Histiocytosis in Children. RadioGraphics. 2008 May 1;28(3):707–26.
- Zaveri J, La Q, Yarmish G, Neuman J. More than Just Langerhans Cell Histiocytosis: A Radiologic Review of Histiocytic Disorders. RadioGraphics. 2014 Nov 1;34(7):2008–24.