48uep6bbphidcol2|ID
48uep6bbphidvals|3038
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Sarcina ventriculi is a gram-positive, non-motile, chemo-organotrophic, obligate anaerobic microorganism that grows in the acidic environment with carbohydrate fermentative metabolism.1 Characteristicmorphology includes spherical shape, 1.8-3 µm individual size, occurs in tetrads or octet (cell division in two or more planes of growth) and refractile nature that can mimic vegetable matter due to birefringent cellulose coating.Numerous cases of fatal disease in animals have been attributed to this organism in the veterinary literature. In humans, it was first observed and described by John Goodsir in 1842. Only a few cases of human infections are reported since then.1
Most cases have dyspepsia, abdominal pain, nausea, vomiting and food residue on endoscopy.2 Few cases were associated with life-threatening complications such as emphysematous gastritis, peritonitis and gastric perforation.2 This association draws attention towards microbial pathogenicity in humans. Hence, in this case series, we review clinical and pathologiccharacteristics of Sarcina infected patients.
Methods
This retrospective case series included patients who were identified with Sarcina infection. We reviewed the pathology database of our tertiary care centre to identify patients with Sarcina ventriculi on endoscopic mucosal biopsies between December 2015 and December 2019. Total 13 cases of Sarcina ventriculi were identified and included in the case series. Medical records of thirteen patients with Sarcina ventriculi organism were reviewed for demographic details, clinical features, imaging results, diagnostic process, and management
Results
Clinical characteristics
The clinical features of all patients are elucidated in Table 1. Of the 13 patients,11 were male and 2 were female. The age of the included patients ranged from 24 years to 73 years. Presenting symptoms included: epigastric discomfort (n=6), epigastric pain (n=4), anorexia (n=6), nausea and vomiting (n=5), constipation (n=2), diarrhoea (n=2) and weight loss (n=3).
Of these 13 patients, 10 had GOO and 2 of the remaining 3also had food residue on OGD suggestive of delayed gastric emptying. The location of GOO was the antrumin 7 and duodenum in 3 patients. Sixpatients had malignant GOO (4- Carcinoma stomach, 2- carcinoma duodenum). Causes of benign GOO included Hpylorigastritis (n=1), eosinophilic gastritis (n=1) and duodenal ulcers (n=2). One patient with duodenal ulcers had a history of prolonged NSAID use. 4 Patients had underlying comorbidities. None of the patients had past gastric surgery or procedure. ( Table 1)
All patients showed the presence of Sarcina ventriculi on the luminal mucosal surface of epithelium with their characteristic morphology on H and E staining. One patient had concomitant H. pylori infection. There was no common pathological finding of the gastric and duodenal mucosa that could be attributed to Sarcina (Figure 1 and 2).
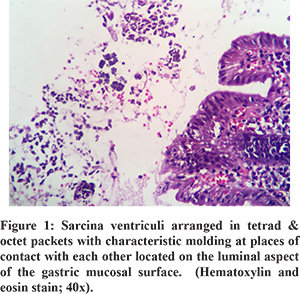
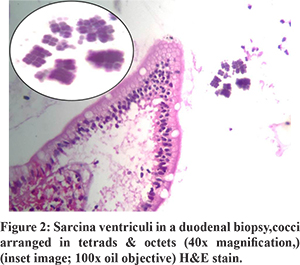
All patients with benign underlying disease (n=7) responded well to antibiotics (metronidazole alone in 10, ofloxacin + metronidazole in 2, Anti H. pylori regimen in 1) and proton pump inhibitors (PPI) with symptomatic improvement in all by 3 weeks. The clinical follow-up duration ranged from 2 to 12 months. Follow up OGD was available in 5 of 7 patients with benign aetiology. Two of these patients had evidence of food residue in the stomach on follow up OGD. One patient had recurrence of symptoms at 3 months. OGD at 3 months showed mild antral gastritis with gastric residue. Biopsy showed Sarcina ventriculi and the antibiotic course was repeated. OGD at 6 months showed no evidence of Sarcina ventriculi. The patient with perforated duodenal ulcer was managed conservatively with nasojejunal feeding as the patient was unwilling for surgery. Repeat OGD at 1 month showed persistence of the perforated ulcer. Biopsy revealed the presence of Sarcina and no evidence of malignancy. The patient was later diagnosed with metastatic adenocarcinoma on follow up at 4 months and succumbed to the same. All other patients with malignant GOO had unresectable disease at diagnosis. 1 patient had partial improvement in GOOat 2 weeks with antibiotics. Repeat OGD was not done in patients with malignant GOO. Histology on follow up OGD was available in 4 of the 5 patients. Only one patient showed the presence of sarcina on follow up biopsy. Repeat antibiotic course cleared sarcina.
Discussion
Despite the first description in 1842, only 33 cases of human infections have been documented prior to this study (Table 2). The research on this organism has flourished in recent years as evident by the recent publication surge. If the pathologist is aware of Sarcina, the likelihood of mistaking it for vegetable matter decreases.
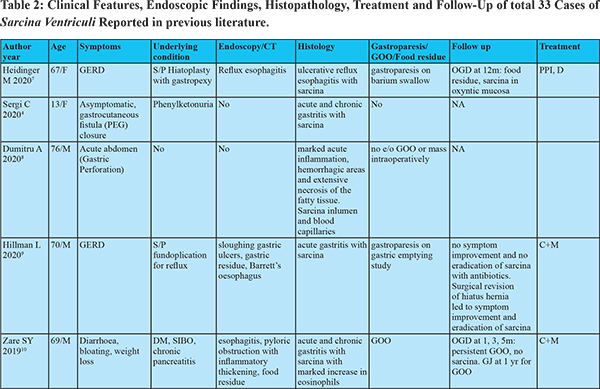
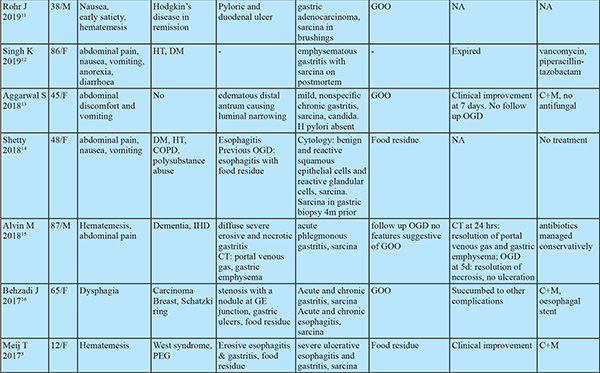
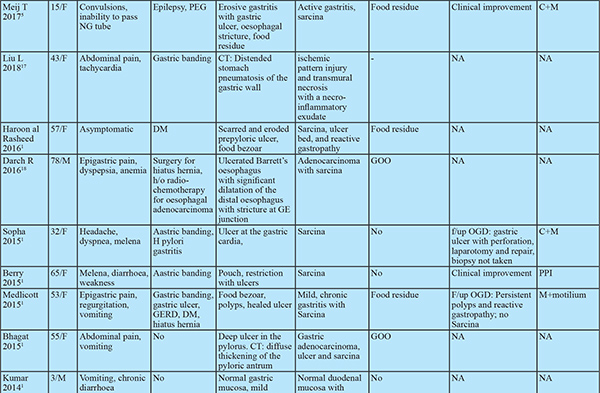
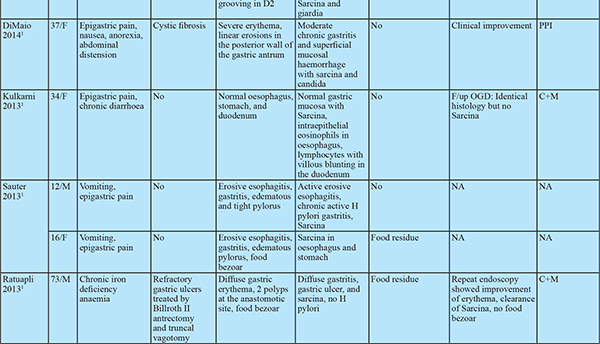
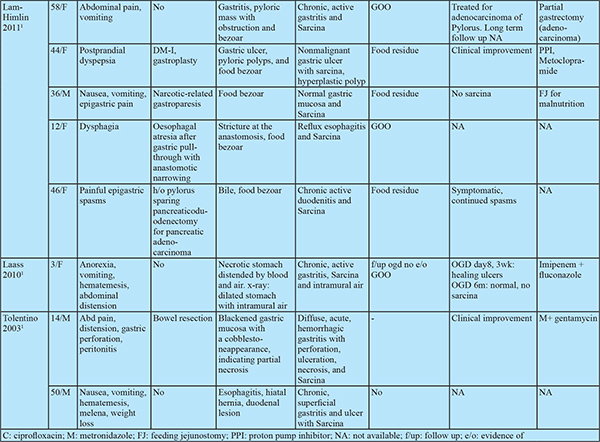
Sarcina hasits natural habitat in the soil, stagnant water, on the surface of cereal seeds and interestingly as one of the major bacterial contaminants isolated from commercially available children’s soap bubbles. Sarcina reaches the gastrointestinal tractbythe ingestion of contaminated food.2,3 Moreover, it has been identified in the faeces of healthy adults.1 Sarcina can be identified in any age group ranging from 3 years to 87 years. While previous reports showed female preponderance, our study had male preponderance. Sarcina has been identified from the stomach (85%), oesophagus (10%) and duodenum (5%) and can lead to complications like emphysematous gastritis or perforation.3 In our case series, Sarcina was found in the stomach and duodenum and not in the oesophagus. Mucosal inflammation and ulceration were seen in all patients. Previous case reports have shown the presence of Sarcina in gastricbiopsy even in asymptomatic patients. Histology however showed acute and chronic gastritis.4 This suggests the presence of mucosal inflammation might cause more symptomatic disease by worsening underlying disease. Sarcina as the cause of this mucosal injury seems less likely as no specific histologic characteristics could be attributed to sarcina. 12 of the 13 patients had food residue on OGD suggesting an association with delayed gastric emptying. Of the 33 cases in prior literature, 8 had GOO and an additional 9 had food residue on OGD (gastroparesis confirmed in 2 by gastric emptying studies). In patients with food residue on OGD, 3 patients had diabetes mellitus and 1 patient had narcotic bowel syndrome. 12 had alteredanatomy of which 8 had food residue on OGD and one had an oesophagal stricture. This might suggest a predisposition to sarcina colonization by probable delayed gastric emptying and altered gastric anatomy. Delayed gastric emptying provides carbohydrate and other fermentative substrates from food to grow Sarcina. They can survive in the low pH of the stomach that provides an ideal environment for Sarcina over growth and subsequent passage of organism from the stomach to duodenum as the duodenum does not have a highly acidic environment.5,6
Meij et al. reported Sarcina in oesophagal biopsyin patients with an oesophagal stricture. Severe stenosis provides an optimal anaerobic condition for Sarcina growth.6 Occasionally, Sarcina coinfected with H. Pylori and Candida. The coinfections with H. Pylori are due to existing environmental conditions and coinfection with candida is associated with GOO.6 None of the patients in our series presented with emphysematous gastritis or gastric perforation. 3 patients presenting with emphysematous gastritis and 2 with gastric perforation has been documented in previous literature. Follow up OGD in 2 patients showed no GOO or food residue on endoscopy. However gastric emptying studies were not done. All patients developing emphysematous gastritis were elderly except one with altered gastric anatomy. Whether mucosal injury predisposes to colonisation by sarcina and complications like emphysematous gastritis cannot be inferred convincingly but prompts towards a possible association. In our series sarcina was found on the mucosal surface and tissue invasion was not seen. However, sarcina has been found in capillaries of the gastric wall and gastric tissue in patients with emphysematous gastritis and gastric perforation. This association requires consideration as management of dysmotility and/ or antibiotic treatment can prevent likely complications especially in elderly patients. All patients with benign aetiology in our study had symptomatic improvement with antibiotics, PPI and prokinetics. However, there was no comparative non-intervention group. The available literature is split regarding the use of antibiotics. Some patients have shown improvement only with the use of PPI and prokinetics even in the presence of ulcerated mucosa while one patient showed nonresponse even after treatment with antibiotics but responded after correction of gastric anatomy and physiology. Till further data is available it might be prudent to treat with prokinetics in non-ulcerated disease and add antibiotics in patients with ulcerations on OGD or inflammation on biopsy. If this fails, surgical correction of delayed gastric emptying (e.g. gastrojejunostomy in GOO) should be attempted.
We did not confirm Sarcina by PCR sequencing of the 16s rRNA gene. However, histologic appearance is characteristic and cannot be confused with other causes.
Conclusion
In conclusion, GOO and possibly gastroparesis is the predisposing factor for Sarcina infection. Though the microorganism is unlikely to cause any direct mucosal injury, GOO and the presence of mucosal injury might predispose to complications. The pathologist must be aware of it and if identified, it must be documented in the report to warrant further work-up and treatment.
References
- Al Rasheed MRH, Senseng CG. Sarcina ventriculi: Review of the literature. Archives of Pathology &Laboratory Medicine. 2016;140(12):1441-5.
- Gaspar BL. The significance of Sarcina in routine surgical pathology practice. Apmis. 2016;124(6):436-43.
- Lam-Himlin D, Tsiatis AC, Montgomery E, Pai RK, Brown JA, Razavi M, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. The American journal of surgical pathology. 2011;35(11):1700.
- Sergi C, Lam J, Persad R. Clostridium ventriculi Infection in a Child with Phenylketonuria. Annals of Clinical & Laboratory Science. 2020;50(1):134-5.
- de Meij TG, van Wijk MP, Mookhoek A, Budding AE. Ulcerative Gastritis and esophagitis in two Children with Sarcina ventriculi Infection. Frontiers in medicine. 2017;4:145.
- Sauter JL, Nayar SK, Anders PD, D’Amico M, Butnor KJ, Wilcox RL. Co-existence of Sarcina organisms and Helicobacter pylori gastritis/duodenitis in pediatric siblings. Journal of clinical & anatomic pathology (JCAP). 2013;1(1).
- Heidinger M, Gorkiewicz G, Freisinger O, Brcic I. Ulcerative reflux esophagitis associated with Clostridium ventriculi following hiatoplasty–is antibiotic treatment necessary? A case report. Zeitschrift für Gastroenterologie. 2020;58(05):456-60.
- Dumitru A, Alius C, Nica AE, Antoniac I, Gheorghia D, Gradinaru S. Fatal outcome of gastric perforation due to infection with Sarcina spp. A case report. IDCases. 2020;19:e00711.
- Hillman L, Jeans P, Whiting P. Gastrointestinal: Sarcina ventriculi complicating gastric stasis. J Gastroenterol Hepatol. 2020 Apr;35(4):527.
- Zare SY, Kubik MJ, Savides TJ, Hasteh F, Hosseini M. A rare case of Sarcina ventriculi diagnosed on fine needle aspiration. Diagnostic Cytopathology. 2019;47(10):1079-81.
- Rohr JM, Eidem ME, Lele SM. First report of Sarcina ventriculi in a pyloric and duodenal brushing specimen. Cytopathology. 2019;30(5):563-4.
- Singh K. Emphysematous gastritis associated with Sarcina ventriculi. Case reports in Gastroenterology. 2019;13(1):207-13.
- Aggarwal S, Tyagi R, Selhi PK, Garg A, Sood A, Sood N. Coinfection of Sarcina ventriculi and Candida in a patient of gastric outlet obstruction: An overloaded pyloric antrum. Diagnostic Cytopathology. 2018;46(10):876-8.
- Shetty NU, O’Connell J, Oshilaja OO, Patil DT, Procop GW, Sturgis CD. First documented case of Sarcina in esophageal brushing cytology. Diagnostic Cytopathology. 2018;46(10):886-7.
- Alvin M, Al Jalbout N. Emphysematous gastritis secondary to Sarcina ventriculi. Case Reports. 2018;2018:bcr-2018-224233.
- Behzadi J, Modi RM, Goyal K, Chen W, Pfeil S. Sarcina ventriculi as an unknown culprit for esophageal stricturing. ACG case reports journal. 2017;4.
- Liu L, Gopal P. Sarcina ventriculi in a Patient With Slipped Gastric Band and Gastric Distention. Clinical Gastroenterology and Hepatology. 2018;16(4):A25-A6.
- Darch R, Harrison J, Rashid M. Sarcina ventriculi bacteria in stomach and duodenum of a patient with gastrooesophageal obstruction by adenocarcinoma. Universal Surg. 2016;4:1-3.