|
Nair Sujit Padmanabhan, Prasanta Debnath, Sanjay Chandnani, Parmeshwar Junare, Shubham Jain, Ravi Thanage, Pravin Motilal Rathi Department of Gastroenterology, Topiwala National Medical College and B.Y.L Nair Hospital, Mumbai, India.
Corresponding Author:
Dr Nair Sujit Padmanabhan Email: sujitnair12345@gmail.com
48uep6bbphidcol2|ID 48uep6bbphidvals|3023 48uep6bbph|2000F98CTab_Articles|Fulltext Multiple primary malignant neoplasm (MPMN) occur frequently than can be explained on the basis of random chance. They are divided into two groups; synchronous and metachronous. Metachronous cancer is detected after 6 months of the first diagnosis and synchronous cancer within 6 months of the first diagnosis. The incidence of developing synchronous gastrointestinal malignancies is noted to rise with increasing age as the long-term survival for each cancer is increasing, more sophisticated treatments available and better application of screening programs. Synchronous cancers are clinically relevant with regard to their diagnosis and treatment.1 The frequency of multiple primaries is reported to be in the range of 2-17%.2 Synchronous cancer has an incidence of 3.4% in gastric cancer (GC)patients. The most common type of synchronous cancer in GC patients is colorectal cancer (CRC).3 Most synchronous cancers consist of two lesions, rarely three or even four. In an exceptional case, Kibera et al. reported a case with 7 simultaneous colon cancers.4
Case Report
A male patient aged 72 years presented with recurrent non bilious vomiting, jaundice, itching, and anorexia and weight loss of 10 kg for 2 months. He developed increased stool frequency, which was small in volume, non-bloody for 15 days. Physical examination of the patient revealed pallor, icterus, cachexia, and a distended gall bladder. His background and family history were not significant. He was a chronic smoker and had a history of alcohol abuse for 20 years. There was no history of any chronic illness in the past. Blood investigations revealed a hemoglobin concentration of 9.2g/dl, leucocyte count of 12000/ul and a blood group O.Liver profile demonstrated total bilirubin 6 mg/dL, direct bilirubin 4 mg/dL, aspartate aminotransferase 124 U/L, alanine aminotransferase 119 U/L, and alkaline phosphatase 412 international U/L.Stool occult blood test was positive.Esophagogastro-duodenoscopy showed a rigid stomach with uniformly thickened gastric folds from fundus to pylorus with few ulcerations creating a challenging duodenal intubation (Figure 1A and B). The first, second part of duodenum and ampulla was normal on esophagogastro-duodenoscopy. Multiple gastric biopsies were taken with conventional forceps that revealed a poorly differentiated adenocarcinoma with signet-ring cell appearance and negative for Helicobacter pylori (Figure 3A and B). Multislice Computed tomography (MSCT) revealed marked wall thickening of the stomach with peri gastric lymph nodes, an abrupt cut off at distal common bile duct (Figure 2A and C),wall thickening of transverse colon, splenic flexure and rectum (Figure 2B and D). ERCP (endoscopic retrograde cholangiopancreatography) scope traversed with difficulty across the pylorus. A brush cytology and a distal common bile duct biopsy was taken after a precut sphincterotomy that revealed a moderately differentiated adenocarcinoma (Figure 4B). Colonoscopy revealed an ulcerative, partially obstructing circumferential mass in the rectum and an ulceroproliferative mass at splenic flexure that could not be traversed, with a normal intervening mucosa (Figure 1C and D). Carcinoembryonic antigen (CEA) level was 6ng/ml (normal range 5ng/ml). Thecolonoscopic biopsies from rectum and splenic flexure showed a poorly differentiated adenocarcinoma with signet ring appearance (Figure 3C and 4A). The immunohistochemistry panel demonstrated the gastric neoplasm to be cytokeratin (CK)7 positive and negative for CK20 and CDX2 while the colonic neoplasm was positive for CK7/CK20/CDX2 (Figure 5A-E). The IHC proved the gastric and colonic lesions to be separate cancers and not metastatic lesions. He was diagnosed as a case of synchronous gastrointestinal neoplasm.
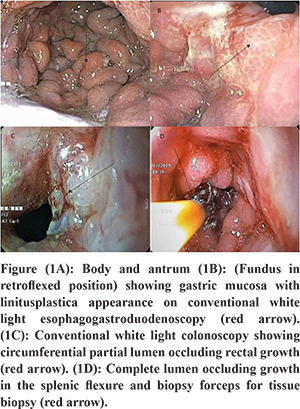
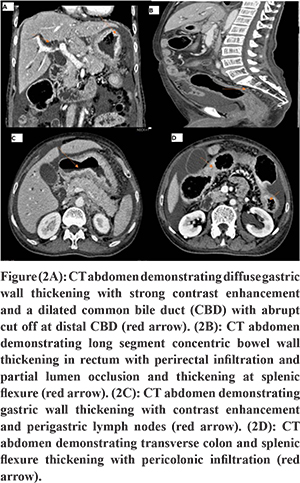
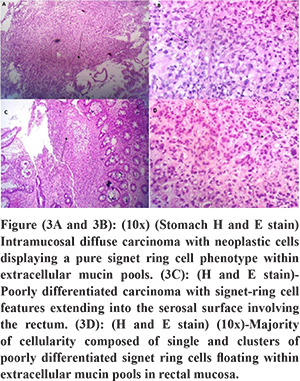
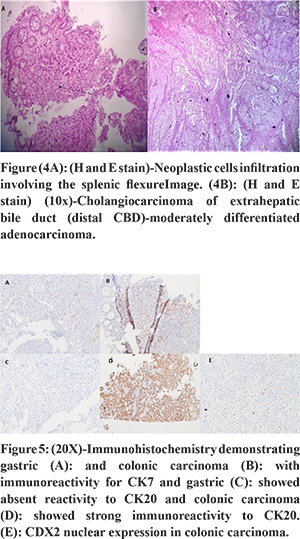
Discussion
In patients with a gastric neoplasm there is also an increased risk of second tumor, mainly during the first year after the diagnosis of the index neoplasm, with an important decrease in survival5. The incidence of synchronous or metachronous cancer in gastric carcinoma is 3.4%6. Synchronous triple neoplasms of gastrointestinal tract involving the stomach, common bile duct and colon is extremely rare. The median age at diagnosis of gastric cancer is 67 years (range, 37-84 years), 69 years for patients with synchronous tumors versus 60 years for those withmetachronous tumors7. Patients with synchronous GC and CRC present in older age, with higher comorbidities, and advanced cancer stage than with metachronous CRC and GC. The incidence of synchronous cancers is significantly higher in men than in women, and it also generally increased with age8. The presented patient clinical profile coincided with the epidemiological characteristics described in the literature. Signet ring cell cancer (SRCC) is a rare entity representing less than 14% of GC and 1% of CRC9. It is a subtype of poorly cohesive carcinoma (WHO classification)10. Due to decreased expression of E-cadherin, the signet cells do not adhere to each other leading to migration and invasion of adjacent tissues. In patients with gastric cancer diagnosed with MMPN, most frequent locations of second neoplasms are, in order of frequency: colorectal (19%), uterus and ovarian (16%) and breast (13%)5. Kibera et al. showed that the incidence of synchronous cancer with multiple gastric cancers was statistically higher than that of synchronous cancer with single gastric cancer11. In the 23 year study by Malgorzata GC patients with second primary were more commonly blood group O (50%) and blood group A (27%) and a significant association between older age and blood group O prevailed. Gastric cancer without second primary was associated with blood group O12. Another study found that 27% of patients with Gastric cancer with second primary was blood group O and the rest were blood group A who were Helicobacter pylori positive13. Our patient was in the extremes of age with blood group O and Helicobacter pylori negative. There is need to include gastric cancer patients with blood group O as controls in the study of synchronous neoplasms. The dominant symptoms in the patient addressed pointed towards gastric etiology and obstructive jaundice and the presence of colonic disease was incipient. In most of cases of patients with synchronous cancers, there was the dominance of obstructive colon symptoms, while the gastric disease symptoms were moderate or overlapping14. Recent studies indicate that after a diagnosis of GC, older patients in particular should be investigated for second malignancies. The detection of synchronous GC and CRC can be obtained by easily available diagnostic methods. Synchronous CRC was detected with a high prevalence of 4% by Saito et al. when colonoscopy was selected as the screening method of choice in gastric cancer patients. Saito et al detected synchronous colorectal adenoma (39%) and CRC (4%) in patients of GC who were older than 50 years through screening colonoscopy. Therefore, it is recommended that if possible, a colonoscopy screening is performed before treatment of patients older than 50 years with GC15. The use of colonoscopy in asymptomatic elderly men for CRC is beneficial to identify patients with synchronous colonic neoplasia16. CT colonography is valuable in those patients with stenosis that leads to incomplete colonoscopy. In our case scenario CT colonography guided us in detecting the transverse colon lesion due to an incomplete colonoscopy. A favorable prognosis in early GC but a poor or similar prognosis for SRCC in advanced GC has been reported in literature17,18. Patients with multiple metachronous primary neoplasms showed better survival rates in contrast to patients with synchronous neoplasms19,20. Metachronous gastric and colon cancer is an independent predictor of survival and it had a better prognosis than synchronous cancer21. CDX2 expression is an excellent marker of adenocarcinomas arising in the GI tract, particularly colon and high levels (> 75% positive cells) of CDX2 expression were found almost exclusively in adenocarcinomas of the colorectum22. The CK7-/CK20+ pattern was identified in 65% to 95% of the colorectal adenocarcinomas and gastric cancers are CK7+ in different series. Few cases of colorectal carcinomas demonstrate CK7+ pattern22,23.The immunohistochemistry in our patient differentiated the colorectal cancer from metastatic gastric cancer and directed toward a synchronous malignancy. Patients with colorectal tumors with MSI (microsatellite instability)are usually young and have longer survival times than patients with tumors without MSI and correlated with the tumor location in the proximal colon24. Kim et al. in their study concluded that the presence of microsatellite instability (MSI) in gastric cancer may be a predictor of synchronous gastric and colorectal neoplasms. MSI-H (microsatellite high) GC prevalence in Asians is commonly?<?10% of all GC cases25. The patient in our case had no family history of cancer, was in the extremes of age, had a distal colonic malignancy and capitulated to the aggressive nature of the neoplasm. The case highlights theneed for early diagnosis of synchronous cancer and awareness of clinicians about this aggressive form of tumors and enlightens the role immunohistochemistry in synchronous neoplasms.
References - Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology. 2003;65(2):113-7.
- Coyte, A., Morrison, D.S., McLoone. Second primary cancer risk-the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC cancer. 2014 Dec; 14:272.
- Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG et al. Gastric cancer patients at high-risk of having synchronous cancer. World journal of gastroenterology: WJG. 2006 Apr 28;12(16):2588.
- Kaibara N, Koga S, Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984 Nov 1; 54:1870-4.
- Lundegardh G, Hansson LE, Nyrbn O, Adami HO, Krusemo UB. The risk of gastrointestinal and other primary malignant diseases following gastric cancer. Acta oncologica. 1991 Jan 1;30(1):1-6.
- Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK et al.. Synchronous and metachronous cancers in patients with gastric cancer. Journal of surgical oncology. 2008 Aug 1;98(2):106-10.
- Dinis-Ribeiro M, Lomba-Viana H, Silva R. Associated primary tumors in patients with gastric cancer. Journal of clinical gastroenterology. 2002 May 1; 34:533-5.
- Ikeda Y, Saku M, Kishihara F, Maehara Y. Effective follow-up for recurrence or a second primary cancer in patients with early gastric cancer. British journal of surgery. 2005 Feb 1;92(2):235-9.
- Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014 Jan 1;17(1):43-53.
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. World Health Organization; 2010.
- Kaibara N, Maeta M, Ikeguchi M. Patients with multiple primary gastric cancers tend to develop second primaries in organs other than the stomach. Surgery today. 1993 Feb 1; 23:186-8.
- Lawniczak M, Gawin A, Jaroszewicz-Heigelmann H, Rogoza-Mateja W, Raszeja-Wyszomirska J, BialekA et al. Synchronous and metachronous neoplasms in gastric cancer patients: a 23-year study. World Journal of Gastroenterology: WJG. 2014 Jun 21;20(23):7480.
- Lawniczak M, Starzynska T. [Helicobacter pylori infection in gastric cancer patients]. Pol MerkurLekarski 2002; 13: 103-106 [PMID: 12420337]
- Lei Z, Zhao H, Liang D. Clinical analysis of 13 cases of synchronous gastric and colorectal cancer. The Chinese-German Journal of Clinical Oncology. 2007 Aug 1; 6:331-3.
- Saito S, Hosoya Y, Togashi K, Kurashina K, Haruta H, Hyodo M et al. Prevalence of synchronous colorectal neoplasms detected by colonoscopy in patients with gastric cancer. Surgery today. 2008 Jan 1;38(1):20-5.
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Harford WV et al.Use of colonoscopy to screen asymptomatic adults for colorectal cancer. New England Journal of Medicine. 2000 Jul 20;343(3):162-8.
- Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World Journal of Gastroenterology: WJG. 2015 Oct 28;21(40):11428.
- Chiu CT, Kuo CJ, Yeh TS, Hsu JT, Liu KH, Yeh CN et al. Early signet ring cell gastric cancer. Digestive diseases and sciences. 2011 Jun 1;56(6):1749-56.
- Kim JH, Rha SY, Kim C, Kim GM, Yoon SH, Kim KHet al. Clinicopathologic features of metachronous or synchronous gastric cancer patients with three or more primary sites. Cancer research and treatment: official journal of Korean Cancer Association. 2010 Dec;42(4):217.
- Shin SJ, Park H, Sung YN, Yoo C, Hwang DW, Park JH et al.Prognosis of pancreatic cancer patients with synchronous or metachronous malignancies from other organs is better than those with pancreatic cancer only. Cancer research and treatment: official journal of Korean Cancer Association. 2018 Oct;50(4):1175.
- Watanabe M, Kochi M, Fujii M, Kaiga T, Mihara Y, Funada T et al. Dual primary gastric and colorectal cancer: Is the prognosis better for synchronous or metachronous?. American journal of clinical oncology. 2012 Oct 1;35(5):407-10.
- Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. The American journal of surgical pathology. 2003 Mar 1;27(3):303-10.
- Wang N, Zee S, Zarbo R, Bacchi C, Gown A. Coordinate expression of cytokeratins 7 and 20 defines unique subsets of carcinomas. Applied immunohistochemistry. 1995 Jul 1;3(2):99-107.
- Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7; 260:816-9.
- Kim YB, Lee SY, Kim JH, Sung IK, Park HS, Shim CS et al. Microsatellite Instability of Gastric and Colorectal Cancers as a Predictor of Synchronous Gastric or Colorectal Neoplasms. Gut and liver. 2016 Mar;10(2):220.
|