48uep6bbphidcol2|ID
48uep6bbphidvals|3005
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Anorectal malformations (ARM) are one of the commonest congenital anomalies. Pouch colon is a regional type of ARM found exclusively in the Asian subcontinent. It comprises around 5-30% of all anorectal malformations in parts of North India.1 It is characterised by a pouch-like dilatation of variable length of the colon associated with an absent anal opening and a colovaginal or colovesical fistula. The etiopathology of this entity is still not established. The management protocol of various types of the pouch is still under the process of standardization. The immediate and long-term results and prognosis also differ from the other common types of ARM. There have been very few studies on histopathological aspects of pouch colon.2-8 We undertook this prospective study to establish the histopathological characteristics of pouch colon and throw some light on this pathology’s etiopathogenesis, management, and prognosis.
Materials and Methods
We conducted a prospective comparative study at a tertiary care centre from July 2015 till June 2018 after ethical clearance was obtained from the Institutional Ethics Committee. All the neonates who were confirmed to have a pouch colon on exploration were included in the study as cases. All the neonates with high ARM who did not have a pouch but were explored to construct high sigmoid colostomy served as a control population. All patients were explored through a left hockey stick incision which was extended as a lower skin crease incision if required.
The presence of pouch colon was diagnosed and confirmed by the typical gross anatomical characteristics such as pouch-like dilatation of the involved colon with a sudden transition to the normal colon, shortened proximal colon, lack of haustrations and appendices epiploicae, poorly developed taenia, and abnormal pattern of vascular supply to the pouch. The type of pouch according to the Rao classification was noted.
As per our institutional policy, all cases of pouch colon were managed by division of colovesical/colovaginal fistula, pouch excision, and terminal colostomy or ileostomy as per the type of pouch. The excised pouch was sent for histopathological examination. All the controls were diagnosed as high ARM based on clinical examination and cross table prone, lateral X-ray film and were explored by left hockey stick incision to construct a high sigmoid colostomy. Approximately 1 cm width of tissue, including the entire circumference of the sigmoid colon from the colostomy site, was sent for histopathological examination.
The biopsy specimens were 10% formalin-fixed and embedded in paraffin blocks, followed by staining with Haemotoxylin and Eosin and Masson’s trichrome. Microscopic evaluation of all the layers of the pouch (mucosa, submucosa, muscularis mucosa, inner circular muscle layer, outer longitudinal muscle layer, and the ganglion cells) was done. Immunohistochemistry with calretinin for nerve cell bodies was done for the evaluation of ganglion cells. The findings were compared between the cases and controls, and analysis was done by the significance of the difference between the proportion of patients in cases and controls, surmised by the Chi-Square test. The results were considered significant at p<0.05. An attempt was also made to compare the findings between the various types of pouches. However, the small number of type 1 and 2 pouches made the exercise unproductive. Statistical software SPSS version 16.0 was used for analysis of the results.
Results
A total of 25 patients with pouch colon and 25 patients of high ARM were included in the study. There were 21 males and 4 females; M: F ratio- 5:1 in the CPC group, while there were 23 males and 2 females in the high ARM group. The mean age at surgery was two days in both groups.
Three of the patients had a perforated pouch found on exploration; in 2 out of these, the preoperative x-ray showed pneumoperitoneum. All 3 of these patients had a delayed presentation, ranging from day 3 to day 5 of life.
The distribution according to the type of pouch was: 16 patients with Type 4 pouch, 3 patients with Type 3 pouch, one patient with Type 2 pouch, and 5 patients with Type 1 pouch.
The histopathological features of the CPC and normal colon are summarised in Table 1. Deviation from the normal histopathology was found in all four layers of the pouch. The mucosa showed necrosis, focal erosions, inflammation, and haemorrhage. Necrosis was present in all 25 (100%) cases(p= 0.000), focal erosions were seen in 19/25 patients (76%) (p=0.001) and inflammation and haemorrhage was found in 23/25 cases (92%)(p=0.001). (Figure 1) None of the normal colon specimens had any mucosal changes like focal erosions, necrosis or inflammation, and haemorrhage.
The muscularis mucosa in the pouch colon patients revealed significant differences from the normal controls. Importantly, substantial fibrosis was present in 20/25 cases (80%) of pouch colon specimens, whereas none of the controls had muscularis mucosa fibrosis (p=0.001). (Figure 2)
The submucosa revealed congestion and haemorrhage, widening, fibrosis, and presence of
lymphoid follicles. Congestion and haemorrhage were present in all 25(100%) pouch colon specimens as compared to none of the controls (p=0.000). (Figure 1) Submucosal widening was present in 22/25 patients with pouch colon (88%), whereas three patients of Type 4 pouch colon had no widening. None of the controls demonstrated any submucosal widening (p=0.001). Fibrosis, as was demonstrated by excessive staining on Masson’s Trichrome was seen in the submucosa, being present in 22/25 patients(88%)compared to being present in none of the control(p=0.001). (Figure 2) Finally, a proliferation of lymphoid follicles was seen in the submucosa of 96% ( 24/25) of CPC, being absent in all controls (p=0.001).
The inner circular and outer longitudinal layers displayed a wide variety of histological abnormalities (Figure 3). The inner circular muscle layer (ICM) was normal in 11/25(44%) cases, atrophic in 10/25 cases (40%), and hypertrophic in 4/25 cases (16%) of cases. Nevertheless, this difference was not statistically significant (P=0.067). The pattern of atrophy/ hypertrophy was also not limited to any particular variant of the pouch colon, being distributed across all variants.
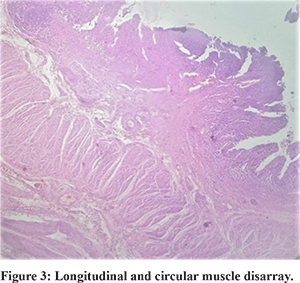
The typical continuous circular muscle fibre arrangement in ICM was seen only in 4/25 patients (16%). In 9 patients (36%), this continuous muscle fibre arrangement was disrupted. Abnormal decussating crisscross fibres were present in 1 patient (4%), whereas constriction bands were present in 4 patients (16%). Additionally, seven patients had a combination of irregularities such as disrupted fibres with crisscross bands (3 patients- 12%), disrupted fibres with constriction bands (3 patients, 12%), and crisscross decussating fibres along with constriction bands (1 patient, 4%). None of the controls had an abnormal pattern of muscle fibre arrangement. This difference in the ICM fibre arrangement pattern was statistically significant between the two groups (p =0.001).
The outer longitudinal muscle layer (OLM) displayed similar results. The layer was normally developed in only 11 patients (44%), was atrophic in 10 patients (40%), and hypertrophic in 4 patients (16%). This was not statistically significant and also showed no correlation with the anatomical Type of pouch. Abnormal patterns of muscle fibre arrangement was seen in OLM layer also. Only 5 patients (20%) had a normal continuous muscle fibre arrangement. In 13 patients(52%), the OLM fibres were disrupted, and 1(4%) patient showed constriction bands. The rest of the cases (6 patients, 24%) had a combination of anomalies, including disrupted layer with decussating crisscross bands (2 patients, 8%), disrupted layer with constriction bands (3 patients, 12%), and decussating bands with constriction ring (1 patient, 4% ). The difference in OLM abnormal fibre arrangement between the cases and controls was statistically significant (p=0.032).
Fibrosis was seen in both ICM and OLM layers, present in 22/25 in ICM and 20/25 in OLM layer, while absent in all controls [p=0.001, p=0.001]. (Figure 2)
21/25 patients ( 84%) of the cases showed a normal number of calretinin stained, ganglion cell bodies in the submucosa or myenteric plexus compared to 22/25(88%) in controls. This finding did not differ significantly between the two groups (P=0.891). (Figure 4)
In our study, all patients underwent pouch excision with terminal colostomy/ileostomy followed by an Abdominoperineal PSARP later. Though increased stool frequency was commonly seen, especially in type I pouch colon, but the often reported problems of constipation, stasis, incomplete evacuation in cases of pouch preservation, and colporrhaphy were seen in none of our cases.
Discussion
Congenital pouch colon is now well recognised as a regional variant of anorectal malformations in the Krickenback Classification.9 It has been mainly reported from the Northern part of the Indian subcontinent, where it can comprise 5-30% of all ARM,1,10 but is sporadic in other parts of the world. Some unique anatomical features characterise it, including a sudden pouch-like dilatation of the involved colon, the sudden transition from normal colon to the pouch without any transition zone, absent haustrations, appendices epiploicae and taenia, and a shortened uninvolved colon.1 It has been classified into five types based on the length of the proximal uninvolved colon by Rao et al. and modified later by Saxena and Mathur as following:11,12
- Type I: Normal colon is absent, and the ileum opens directly into the colonic pouch.
- Type II: The ileum opens into a short segment of the cecum which then opens into the colonic pouch.
- Type III: Presence of a significant amount of normal colon, including ascending and transverse colon, between the ileum and the colonic pouch
- Type IV: Presence of near-normal colon with only the terminal portion of the colon (rectum and a varying portion of the sigmoid) converted into a pouch.
- Type V: Double colonic pouch separated by an intervening area of normal colon.
The etiopathogenesis of this entity is still not fully understood, though many theories have been proposed, including the vascular insult theory, chronic obstruction theory, interference of hindgut growth and migration theory, altered hindgut stimulation theory, faulty rotation and fixation theory etc.1 Presently, based on the histology findings till date, the vascular insult theory seems most plausible. Similarly the management of CPC is not standardised, with some authors recommending preservation of the pouch and its use in subsequent pull through procedure after coloplasty, specially in Type I and II CPC.11,13,14,15 However, it was soon observed that the long term results of coloplasty were variable with some authors describing redilatation of the tubularised pouch and other long term problems.1,16 The functional absorptive capacity of the retained pouch mucosa, either as coloplasty or as a mucosal graft is still not entirely clear, though it is believed to be decreased.1 For these reasons some authors recommend complete excision of the pouch.2,4,5,16,17
An attempt to study the histopathology of the pouch has been made in the past few years, to find answers to these questions of etiology and management. The initial studies reported a normal colon wall.18,19,20 However , later studies discovered the abnormalities in various layers of the pouch.21 Wakhlu et al. in 1996 discovered the pouch to have a thinned out musculature and absent ganglion cells in 2 out of 17 patients.14 Tyagi et al. also reported on the muscle layer abnormalities and paucity of ganglion cells.5 Further studies have emphasised structural and functional abnormalities in all the layers- mucosa, submucosa, inner circular and outer longitudinal muscle layer.2-8 In contrast to the earlier studies, our study found significant aberrations in all the layers of the pouch.
The majority of studies have concentrated on the defects in the muscular layers of the pouch only which are important as dysmotility and dilatation are significant problems in a preserved pouch.4,5,14 However our study found the mucosal layer also to be gravely affected in the pouch specimen. In addition to inflammation and haemorrhage, we found focal erosions and necrosis of mucosa as a significant finding. This would significantly decrease the functional absorptive surface in the pouch. On one hand this lends credence to the vascular insult theory, and on the other hand it also explains why babies with retained pouch or window colostomy continue to have poor absorption and growth retardation. Some authors have described a technique of ‘pouch colon patch graft’ where an attempt is made to retain some of the absorptive surface of the pouch by using it as a patch on the pulled through ileum, especially in Type IV pouch where no normal colon is present.21 In the light of findings of mucosal necrosis and erosions as found in our study, it is conjectured that such procedure may not really fulfil its purpose of increasing the absorptive surface and long term results need evaluation.
The submucosal layer also revealed multiple anomalies, most prominent being widening, congestion and inflammation along with proliferation of lymphoid follicles. Similar findings have been reported by Gangopadhyaya et al. and Udawat et al.2,7 The noteworthy point brought out in this study is that the submucosal widening is the result of both increased connective tissue (fibrosis) as well as increased edema, congestion and inflammation reflected as increased lymphoid follicles.
Abnormalities in the ICM and OLM layers have been documented in almost all studies, though of differing patterns.2-8 The most substantial finding in our study as well as previous studies is the disruption of normal, organized muscle fibre arrangement and various abnormal patterns of the muscle fibre arrangement including muscle layer disruption, constriction bands, decussating crisscross fibres or any combination of these. Also significant fibrosis as demonstrated by Masson’s trichrome stain was found in both OLM and ICM layers. Though some studies have reported an additional muscle layer inside the ICM, this was not found in our case.4,5 We also found that both the ICM and OLM may be either atrophic or hypertrophic in around 50% of cases. The significance of this atrophy and hypertrophy is a matter of debate because it does not correlate consistently with either the type of pouch or any other specific histological feature. On the basis of this study, we feel that it is not the type of abnormal pattern that is important rather than the loss of regular arrangement of muscle fibres. We hypothesize that all these abnormal patterns of muscle arrangements and hypertrophy/atrophy are a marker of dysfunctional tissue and reason for dysmotility, stasis and abnormal pouch function. The presence of fibrosis in the muscle bundles indicates an irreversible damage to the muscular wall that may contribute to the colonic wall dysmotility in cases of CPC. Our study also discovered extensive inflammatory infiltrates in the muscle layers.
Ganglion cells have been assessed by H& E staining in previous studies and found to be deficient.3,5,7 Our study is the first one using calretinin immunohistochemistry for ganglion cell assessment and using this we found no difference in the number of ganglion cells in the myentric plexus between the pouch colon and the control group. This is a new and significant finding as it indicates that dysmotility appears to be the result of structural deficiencies in the colonic wall rather than defect in neural signalling.
In this study, all the cases were of neonates, whereas most previous workers have analysed specimens of pouch ranging from new-born to 4-5 years of age.2-8 As such this study uniquely highlights these histological findings which represent the inherent developmental anomalies in the pouch without any confounding effect of any kind of intervention or growth.
The above findings indicate that the pouch in CPC has abnormalities in all histological layers and hence is a developmentally and functionally abnormal tissue possibly with poor absorptive function and motility, as a consequence of these histological anomalies. It is possible that retaining the pouch by coloplasty or colorrhaphy or even as a mucosal patch may cause continuing or recurrent problems due to these inherent defects. In the light of these findings, it may be prudent to excise the pouch in CPC as it is a pathologically aberrant tissue. The problems of ileal or short colonic pull through may need to be addressed by some alternative means such as creation of a reservoir, rather than by coloplasty or colorrhaphy. Few case reports in the recent times support this thought. Chadha et al. have recently reported three patients who presented 2- 10 years after TC and pull-through with massive colonic redilatation and attacks of severe enterocolitis.16 Similar cases have been reported by Wakhlu et al.14 Further studies on detailed long term results and quality of life of children undergoing colorrhaphy/coloplasty are required to accurately assess the outcomes, which can support or reject this suggestion.
In our institution, CPC has been managed in a staged manner. Based on previous complications of coloplasty such as redilatation, persistent dysmotility etc it has been an institutional policy to excise the pouch and perform terminal colostomy/ileostomy as the first stage. Though diarrhoea is an initial problem in Type I pouch, where ileostomy is required, it usually improves with time with neonatal gut adaptation. The second stage pull through is done after allowing adequate time for gut adaptation and only after the stoma output has become manageable in terms of consistency and frequency.
We would also like to draw attention to the finding of extensive inflammatory infiltrate in all layers of the pouch. This finding has been seen previously in few studies.2,3,7 Though the vascular insult theory explains many features such as muscle fibrosis, congestion and mucosal necrosis, such extensive inflammatory reaction is difficult to attribute to vascular compromise alone. It is hypothesised that some environmental infectious or chemical agent exposure may be responsible for the extensive inflammation and further abnormalities such as mucosal erosions. This may also explain the regional predominance of CPC. This possibility needs further research.
Conclusion
CPC is a variant of ARM which has histopathogical abnormalities in all the layers of the colon including mucosa, submucosa, muscularis propria. Most prominent features are severe inflammation involving all layers of the colon, mucosal necrosis and erosions, submucosal fibrosis and widening and muscular fibres disruption, disorganised arrangement and constriction bands. The widespread anomalies in each layer indicate that CPC is pathologically abnormal tissue and retaining it in any form as by coloplasty or colorrhaphy may not be advisable. The widespread inflammatory reaction in all layers of the CPC raises the possibility that apart from vascular insult, some environmental factor may have a role in etiopathogenesis. Further experimental studies would be required to confirm this hypothesis and delineate the exact etiology of this entity.
References
- Saxena AK, Mathur P . Congenital pouch colon. Puri P(ed). Newborn Surgery, 3rd ed, Hodder Arnold,2010; 582-589
- Gangopadhyaya AN, Pandey A, Upadhyaya VD . Congenital pouch colon associated with anorectal malformation-histopathological evaluation. J PediatrSurg 2009;44: 600-606
- Agarwal K, Chadha R, Ahluwalia C . The histopathology of congenital pouch colon associated with anorectal agenesis. Eur J PediatrSurg 2007;12:1-2
- Chatterjee U, Banerjee S, Basu AK. Congenital Pouch Colon: An unusual histological finding. PediatrSurg Int.2009; 25:377-380
- Tyagi P, Gangopadhyaya AN, Maloy B. Pouch colon associated with anorectal malformations fails to show spontaneous contractions but responds to acetylcholine and histamine in vitro. J Pediatr Surg. 2009 ; 44: 2156–2162
- Chadha R, Agarwal K, Choudhury R . The colovesical fistula in congenital pouch colon: a histologic study. J Pediatr Surg. 2008; 43: 2048-2052
- Udawat H, Nunia V, Mathur P, Udawat H P, Gaur K L, Saxena A, Mohan M K. Histopathological and Immunohistochemical Findings in Congenital Pouch Colon: A Prospective Study. Pathobiology. 2017; 84: 202-209
- Agarwal K, Chadha R, Ahluwalia C, Debnath P R, Sharma A, Roy Choudhary SK. The Histopathology of Congenital Pouch Colon Associated with anorectal agenesis. Eur J Pediatr Surg. 2005; 15:102-106
- Holschneider A, Hutson J, Pena A, Beket E, Chatterjee S, Coran A, et al . Preliminary report on the International Conference for the Development of Standards for the Treatment of Anorectal Malformations. J PediatrSurg . 2005; 40: 1521-1526
- Mathur P, Prabhu K, Jindal D. Unusual presentations of pouch colon. J Pediatr Surg. 2002; 37: 1351-1353
- Narasimha Rao K L, Yadav K, Mitra SK, Pathak I G. Congenital short colon with imperforate anus(pouch colon syndrome). Ann Pediatr Surg.1984; 1:159-67
- Saxena A K, Mathur P. Classification of congenital pouch colon based on anatomic morphology. Int J Colorectal Dis. 2008; 23: 635-639
- Mathur P, Saxena A K, Simlot A. Management of pouch colon based on Saxena- Mathur classification. J Pediatr Surg. 2009; 44:962-966
- Wakhlu A K, Pandey A, Wakhlu A, Tandon RK, Kureel SN . Coloplasty for congenital short colom . J PediatrSurg 1996; 31: 344-348
- Wakhlu A, Wakhlu AK. Technique and long term results of coloplasty for congenital short colon. PediatrSurg Int. 2009; 25:47-52
- Chadha R, Bagga D, Gupta S, Prasad A. Congenital pouch colon: massive redilatation of the tubularized colonic pouch following pull through after tubular colorraphy. J Pediatr Surg.2002; 37:1376–1379
- Chadha R. Congenital Pouch colon associated with anorectal agenesis. PediatrSurg Int. 2004;20:393-401
- Chadha R, Bagga D, Malhotra CJ, Mohta A, Dhar A, Kumar A. The embryology and management of congenital pouch colon associated with anorectal agenesis. J PediatrSurg .1994; 29: 439–446
- Vaezzadeh K, Gerami S, Kalani P, Sieber WK. Congenital short colon with imperforate anus: A definitive surgical cure. J Pediatr Surg. 1982; 17: 198–200
- Wardhan H, Gangopadhyay AN, Singhal GD, Gopal SC. Imperforate anus with congenital short colon (pouch colon syndrome). PediatrSurg Int .1990; 5: 124–126
- Ratan SK, Rattan KN. “Pouch colon patch graft”—an alternative treatment for congenital short colon. PediatrSurg Int .2004;20:801-803.