48uep6bbphidcol2|ID
48uep6bbphidvals|3004
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Gastroparesis is a condition characterized by a delay in gastric emptying associated with upper gastrointestinal symptoms, without mechanical obstruction.1 Multiple etiologies govern gastroparesis, however, diabetes, post-surgical complications, and idiopathic causes account for the majority of gastroparesis cases.2 Symptoms of gastroparesis (of varying severity) include early satiety, nausea, vomiting, postprandial fullness, abdominal pain/discomfort, and bloating. Patients with other upper gastrointestinal disorders like functional dyspepsia often experience similar symptoms, posing a challenge in diagnosing gastroparesis. The recommended diagnostic gold standard test for assessing gastric emptying is gastric scintigraphy carried out using a solid meal tagged with a radio-labelled compound like Technitium99m sulphur colloid.3 Treatment approaches of gastroparesis are multimodal, based on etiology, co-morbid conditions, risk factors and/or associated complications. Prokinetics are commonly used for the management of gastroparesis, which offers prompt symptomatic relief.3
Although there is worldwide literature on gastroparesis, there is a paucity of evidence from the Indian milieu. A few Indian studies on type-2 diabetes (T2DM) patients have demonstrated hyperglycemia-mediated delayed gastric emptying4 and poor correlation of delayed gastric emptying with symptoms of gastroparesis.5 However, scarcity of data about contributing risk factors, etiologies, etc that are directive of the demographic and clinical profile among Indian population would offer impelling indication to undertake the present investigation.
Thus, this pan-India study was planned to understand the demographics and clinical profile of patients with gastroparesis. This study was also intended to assess the clinical history, risk factors, complications, impact on the quality of life (QoL), and diagnostic and management practices in Indian patients with gastroparesis.
Methodology
Study design and patient population
This multi-center, cross-sectional, clinico-epidemiological study was conducted between February 2017 and January 2018 across six centres in India (Jaipur, Bhopal [2 centers each], Guwahati, Hyderabad and Ahmedabad). Patients (=18 years) diagnosed clinically based on symptoms of gastroparesis, viz. postprandial fullness, bloating, abdominal pain, nausea, abdominal discomfort, early satiety and vomiting and willing to participate were included in the study. Patients with obstruction of gastric outlet, small bowel or colon, gastro-intestinal haemorrhage or perforation, chronic cardiac, hepatic, neurological and/or renal diseases (as per physician discretion), and pregnant or lactating women were excluded from the study. In addition, patients who could not provide responses to QoL questionnaire and subsequent written authorization were also excluded from the study.
Severity of symptoms was assessed as mild, moderate and severe. Risk factors such as fatty diet, carbonated drinks and fibre diet were noted. Complications such as malnutrition, weight loss, esophagitis and electrolyte disturbances were noted.
Patients underwent assessment of gastric emptying by gastric scintigraphy, using a standard solid meal tagged with radiolabelled Technetium-99m. Four hours imaging technique with scans taken at 0, 1,2 and 4 hours after ingestion of food was used.
Delayed gastric emptying was defined as >60% retention at 2 hours postprandially and/or >10% retention at 4 hours. Correlation of clinical symptoms and percentage positive gastric scintigraphy was obtained. Etiologies of gastroparesis were also noted.
The study protocol was approved by institutional ethics committees. The study was conducted in accordance with the principles of Declaration of Helsinki, International Council on Harmonization Good Clinical Practice (ICH GCP) guidelines, and Indian regulatory guidelines (Indian Council of Medical Research and Indian GCP guidelines). All patients provided written consent in the patient authorization form to participate in the study.
Study endpoints
The primary study endpoints included percentage of patients with confirmed gastroparesis by scintigraphy, and demographic characteristics (average age, gender, BMI, socio-economic status) of patients with gastroparesis. The secondary endpoints included average duration for the development of gastroparesis from the diagnosis of underlying etiology, proportion of gastroparesis patients with various risk factors, co-morbidities and complications. Additionally, current treatment modalities for gastroparesis and QoL (by Patient Assessment of Upper Gastrointestinal Disorders -Quality of life [PAGI-QoL] score) were also assessed.
Study assessment tool (PAGI-QoL)
Disease (gastroparesis)-specific QoL was assessed by PAGI-QoL survey with scoring on a 6-point Likert scale. The questionnaire was administered by physician or designee. It was used to assess the patients’ health related QoL within the last 2 weeks.6 It consisted of 30 items assessing five domains: Daily Activities, Clothing, Diet and Food Habits, Relationship, and Psychological Well-Being and Distress.7,8 The PAGI-QoL provides numerical values for QoL in patients with disordered gut motility.7 Overall PAGI-QoL scores are means of all factors after reversing individual scores; a mean PAGI-QoL score of 0 represents poor QoL while 5 reflects the best QoL.
Statistical methods
No formal sample size calculation was required for this study. Continuous variables were summarized using descriptive statistics: n (number of patients), mean and standard deviation. Summary of categorical data were evaluated using numbers and percentages. PAGI-QoL questionnaire was assessed by 2 sample t-test. All statistical analyses were done using Statistical Analysis System® version 9.4 software.
Results
Overall, 201 patients met the inclusion criteria of this study, which included 115 males (57.2%) and 86 females (42.8%) (mean [SD] age: 46.8 [14.17] years; Table 1). Most of the patients (63.2%) belonged to upper middle class based on Kuppuswamy scale of socio-economic status.
A total of 196 patients underwent the diagnostic procedure of gastric scintigraphy, of which 88 (45.0%) were scintigraphically positive while 108 (55.0%) were only clinically positive. Remaining 5 patients failed to undergo the procedure due to technical/personal reasons (Figure 1).
Dietary pattern and its correlation with gastroparesis symptoms
Dietary pattern analysis revealed that most patients consumed fatty diet (185, 92.0%), fibre rich food (167, 83.1%), excessively brewed tea (139, 69.2%), carbonated drinks (66, 32.8%) and coffee (56, 27.9%) (Table 2). Daily consumption of these foods with more than one serving was noticed maximally for excessively brewed tea, followed by fiber-rich food and fatty diet. Remarkably, a higher number of patients consumed more than one serving of fatty diet compared to fiber rich food and about one-fourth of the total patients consumed at least one serving of carbonated drink per day.
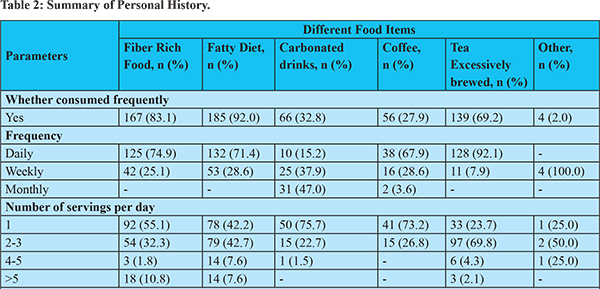
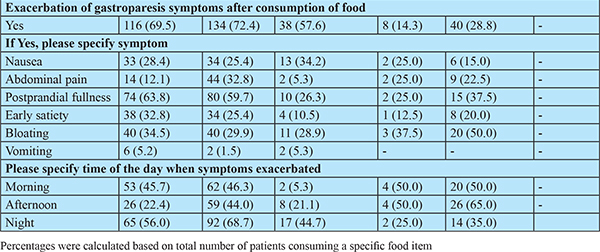
The symptoms of gastroparesis consisted of, but not limited to, postprandial fullness, bloating, early satiety, nausea, vomiting, abdominal pain and discomfort. These symptoms exacerbated in approximately 70% patients after consuming fatty diet (134, 72.4%) and fiber-rich food (116, 69.5%). Excessively brewed tea (40, 23.88%), carbonated drinks (38, 18.9%), and coffee (8, 4%) also exacerbated the symptoms. Presentation of the given symptoms in a specific time-period of a day was largely based upon the type of food consumed. Therefore, these dietary substances constitute one of the major risk factors leading to exacerbation of gastroparesis symptoms (Table 3).
Lifestyle habits
Overall, 38 (~20%) patients reported eating in position other than upright state (Table 4). Relatively higher number of patients reported consuming very spicy food (127, 63.2%) and going to bed immediately after completing the meal (129, 64.2%). In addition, a higher number of patients reported inactive (65, 32.3%) or sedentary (45, 22.4%) lifestyles.
Symptoms of gastroparesis
All enrolled patients presented with one or more of the classical symptoms of gastroparesis. Majority of the patients reported postprandial fullness (152, 75.6%); while approximately half of the patients had bloating (102, 50.7%) and abdominal pain (91, 45.3%). Other symptoms included nausea (83, 41.3%), abdominal discomfort (81, 40.3%), early satiety (76, 37.8%) and vomiting (36, 17.9%). These symptoms were of ‘moderate severity’ in most of the patients (Figure 2).
Complications of gastroparesis
Out of 201 patients, only 24 (~12%) presented with gastroparesis associated complications (Table 3). Weight loss was reported in 13 (6.5%) patients, which developed in a mean duration of 1.3±2.5 years. This was followed by esophagitis (11, 5.5%), with a mean duration of 0.3±0.3 years. Only 1 (0.5%) patient presented with electrolyte disturbances.
Underlying etiology and average duration of gastroparesis development from diagnosis of etiology
The underlying etiology of gastroparesis for most patients was either idiopathic (103, 51.2%) or type 2 diabetes(90, 44.8%), while in few patients it was type1 diabetes mellitus (5, 2.5%) or psychological disorders (3, 1.5%) (Table 5). Like the dietary pattern, the presentation of typical symptoms varied with the type of aetiology (Figure 3). The average duration of gastroparesis (mean±SD) development from diagnosis of T2DM, T1DM and psychological disorders was found to be 58.2±55.82, 133.6±91.53 and 27.3±42.16 months respectively (Table 5).
Co-morbid conditions
About one-fourth (55, 27.4%) of the study patients reported co-morbidity/significant medical history. Most commonly reported co-morbidities were acid-peptic disease (32, 15.9%), hypertension (19, 9.4%), dyslipidaemia(18, 8.9%), and hypothyroidism (10, 4.9%).
Management of gastroparesis
Out of 201 patients, 166 (82.6%) were on pharmacotherapy (proton-pump inhibitors (147, 89.6%), prokinetics (84, 51.2%) and antiemetics (13, 7.9%) (Table 6). A higher proportion of patients (192, 95.5%) were asked to follow specific dietary measures like low-fat, low-fiber diet (163, 84.9%), small frequent meals (160, 83.3%), not to lie down immediately after eating (147, 76.6%), chew well/eat slowly (130, 67.7%) and avoid coffee, tobacco, carbonated drinks, and stress (61, 31.8%).
Quality of life (QoL)
Assessment of QoL score was performed for all 201 patients using PAGI-QoL questionnaire
(Figure 4). The mean±SD total score was found to be 3.6±0.94, suggesting a moderately disturbed QoL in patients with gastroparesis.
Discussion
This study enrolled 201 patients from 6 different centres across the country. The majority of the patients enrolled in our study were aged 31-50 years, with a mean±SD age of 46.8±14.17 years, which is in accordance with several reported studies.1,10 Based on the literature, the onset age of disease was found to be 13 years, but prevalence is reported to be higher in adults above 30 years compared to the younger population. This is likely due to multiple risk factors such as altered dietary and lifestyle habits, reduction in physical activity and development of comorbidities such as diabetes mellitus with age progression.1,10
Unlike the earlier data reporting female preponderance,11 our population was male predominant. This may be due to the unwillingness of female patients to participate in a clinical study due to a lack of awareness and social barriers. The mean ± SD weight (kg), height (m), BMI (kg/m2) and waist circumference (cm) of overall patients were 68.2 ± 9.44, 1.6 ± 0.07, 25.3 ± 3.08 and 93.4 ± 8.89, respectively, which was in line with the earlier reports.1,11,12
Socio-economic status revealed that most patients belonged to the upper-middle class. Moreover, most of the patients in our study had sedentary or extremely inactive lifestyle and altered dietary habits such as fatty diet, fibre-rich food, and carbonated drinks, which are known risk factors for gastroparesis and associated symptoms.
Gastric emptying scintigraphy of a solid-phase meal is considered as the standard for diagnosis of gastroparesis, as it quantifies the emptying of a physiologic caloric meal.13,14 Many studies have demonstrated poor correlation between the classical symptoms and delayed gastric emptying.1,15,17 In this study, gastric scintigraphy was performed on 196 patients, of which only 88 (45.0%) were scintigraphically positive while 108 (55.0%) were only clinically positive. This finding highlights the importance of clinical diagnosis of gastroparesis for early patient identification and appropriate management. This discordance between symptoms and scintigraphy test is probably because factors in addition to slow gastric emptying contribute to symptoms. Furthermore, higher inter-individual and intra-individual variability in gastric emptying rates is considered a limitation of gastric emptying (motor) testing.14 Another cause of discordance could be because gastric emptying even as measured by standardized testing can vary with time in the same patient. So, labelling a patient as having gastroparesis (or not) based on a single point in time may not be accurate.16 It is also not known whether measuring gastric emptying of a standardized meal diet reflects what happens chronically in patients’ real life, which depends on the nature and the quantity of food consumed. More importantly, there is also a discrepancy between liquid and solid-phase gastric emptying, and if only the later were measured, a significant number of patients would have been missed.So, gastric emptying is only one (and perhaps the most extreme) manifestation of disordered gastric motility. Subtle forms of dysmotility within different regions of the stomach may be more responsible for the pathogenesis of symptoms rather than the overall delayed gastric emptying.This fact may account for the lack of correlation between symptoms and results of gastric scintigraphy testing.16
Impairment of gastric function is associated with symptoms that are typically exacerbated due to specific food intake. Therefore, dietary risk factors leading to gastroparesis were also studied in the enrolled population. Maximum patients consumed a fatty diet, followed by fibre-rich food, excessively brewed tea, carbonated drinks, and minimally coffee. These observations corroborate an earlier study that recommended low-calorie food for gastroparesis patients in addition to dietary modifications.18
It is also known that the cardinal symptoms of gastroparesis largely remain the same, irrespective of the etiology, but with varying intensity.3 In our study, the symptoms of gastroparesis were recorded to be of moderate severity in the order of postprandial fullness > bloating > abdominal pain > early satiety > nausea and vomiting, which is consistent with the reported literature.19 However, some studies have reported vomiting in diabetes and abdominal pain in idiopathic gastroparesis as the most common symptoms.3,20,21
Complications associated with gastroparesis based on scintigraphy were monitored in all enrolled patients, of which only ~12% presented with complications that included weight loss, followed by esophagitis and electrolyte disturbances. This observation corresponds with the reported literature.10,22 Timely diagnosis and suitable treatment will aid in preventing complications which in turn would help in improving the QoL of patients.
Major causes of gastroparesis are reported to be diabetes, idiopathic, and post-surgical.23 In this study, we observed the underlying etiologies to be idiopathic and T2DM in the majority of patients, while few patients had T1DM and psychological conditions (anxiety and depression) as a cause for gastroparesis, which is in agreement with the reported literature.23 Furthermore, this is the first of its kind study to investigate the average duration of gastroparesis development from the diagnosis of underlying etiology. The mean ±SD time of gastroparesis development from T1DM incidence was 133.6 ± 91.53 months, whereas after T2DM incidence was 58.2 ± 55.82 months and from psychological disorder was 27.3 ± 42.16 months. This observation might aid in considering prophylactic measures of gastroparesis once a patient is diagnosed with a primary disease.
Among 201 patients, 27.4% presented significant medical history. Of these, most patients reported a history of acid-related disorders. Reported literature has shown a strong co-relation between gastroesophageal reflux disease (GERD) with gastroparesis.20 Additionally, patients also reported comorbid conditions like dyslipidemia, hypertension, and hypothyroidism.24 Thus, the clinician should give due consideration to such comorbid conditions while evaluating patients with gastroparesis.
Management of gastroparesis for its symptoms is crucial to maintain patients QoL. Among 201 patients, 82.6% were given pharmacotherapy. Of the treated cohort, the majority of patients were on proton pump inhibitors (rabeprazole, pantoprazole, omeprazole, esomeprazole, and lansoprazole), followed by prokinetics (domperidone, levosulpiride, itopride, and acotiamide) and antiemetics (ondansetron and metoclopramide). Proton pump inhibitors may help in reducing the symptoms of hyperacidity but may not be adequate in controlling dysmotility symptoms. Prokinetic agents help augment gastric motility, thus are recommended for prompt symptomatic relief in gastroparesis patients.1 According to the American Gastroenterological Association (AGA) guidelines for gastroparesis, the primary therapy indicated for gastroparesis is dietary manipulation, combined with the administration of prokinetic and antiemetic agents.25 Our study recorded only 51% patients on prokinetics, which could be the reason for persisting gastrointestinal symptoms, affecting overall QoL. Therefore, there is a need for knowledge dissemination on the role of prokinetics in addressing the symptoms of gastroparesis for holistic management in such patients.
Gastroparesis has a significant impact on the patients’ QoL, either due to the primary disease or due to associated symptoms and complications. The mean ±SD (Min: Max) total score on the PAGI-QoL was found to be 3.6 ± 0.94 (0.7: 4.8), suggesting a moderate effect of gastroparesis on patients’ QoL. This is in concordance with literature reporting the negative impact of gastroparesis on patients QoL.10,20
Our study has several strengths. This is the first of its kind, pan India study which elucidated demographic and clinical profiling of gastroparesis. Patients of varying ages, socioeconomic status were evaluated. Our study has used standard and validated methods for diagnosis of gastroparesis giving credibility to the results reported. All the questionnaires used in the study were administered to the patients by a physician or a designee, which enabled them to capture information with greater accuracy and confidentiality. This study generated vital information that augmented the understanding and awareness of clinical profiling, diagnosis, and management of Indian patients with gastroparesis. However, this study also has some limitations. This was a single visit study and lacked long-term outcome data. Further, being a non-interventional study, no data were collected on the altered treatment modalities.
Conclusion
This study reiterates the significance of the clinical diagnosis of gastroparesis, especially in patients with diabetes. Gastric scintigraphy studies, the gold standard for diagnosis of gastric emptying, has poor correlation with clinical symptoms of gastroparesis. Gastroparesis moderately affected the QoL of these patients. About half of the patients were prescribed prokinetics, emphasizing the need for appropriate pharmacotherapy using prokinetics for the holistic management of gastroparesis.
References
- Camilleri M, Bharucha AE, Farrugia G. Epidemiology, Mechanisms and Management of Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1–16.
- Clark DW, Nowak TV. Diabetic gastroparesis. Postgrad Med. 1994;95:195–204.
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical Guideline?: Management of Gastroparesis. Am J Gastroenterol. 2013;108:18–38.
- Laway BA, Malik TS, Khan SH, Rather TA. Prevalence of abnormal gastric emptying in asymptomatic women with newly detected diabetes and its reversibility after glycemic control - A prospective case control study. J Diabetes Complications. 2013;27:78–81.
- Anudeep V, Vinod KV, Pandit N, Sharma VK, Dhanapathi H, Dutta TK, et al. Prevalence and predictors of delayed gastric emptying among Indian patients with long-standing type 2 diabetes mellitus. Indian J Gastroenterol. 2016;35:385–92.
- De La Loge C, Trudeau E, Marquis P, Kahrilas P, Stanghellini V, Talley NJ, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: The PAGI-QOL©. Qual Life Res. 2004;13:1751–62.
- De La Loge C, Trudeau E, Marquis P, Revicki DA, Rentz AM, Stanghellini V, et al. Responsiveness and interpretation of a quality of life questionnaire specific to upper gastrointestinal disorders. Clin Gastroenterol Hepatol. 2004;2:778–86.
- Hasler WL, Wilson LA, Parkman HP, Koch KL, Abell TL, Nguyen L, et al. Factors related to abdominal pain in gastroparesis: Contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25.
- Bielefeldt K, Raza N, Zickmund SL. Different faces of gastroparesis. World J Gastroenterol. 2009;15:6052–60.
- Talley NJ, Locke GR, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933–9.
- Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, et al. Treatment of gastroparesis: A multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–83.
- Fass R, McCallum RW, Parkman HP. Treatment challenges in the management of gastroparesis-related GERD. Gastroenterol Hepatol. 2009;5:1–2.
- Emral R. Diabetic Gastroparesis (Gastroparesis Diabeticorum). J Ankara Med Sch. 2002;24:129–36.
- Hasler WL. Gastroparesis: pathogenesis, diagnosis and management. Nat Rev Gastroenterol Hepatol. 2011;8:438–53.
- Ghoshal UC. Pharmacotherapy for Gastroparesis: An Attempt to Evaluate a Safer Alternative. Pharmacotherapy. 2010;16:350–2.
- Pankaj Jay Pasricha, Henry P. Parkman. Gastroenterol Clin N Am, 2015; 44:1-7
- Abid Shah M, Suleman S, Saadatullah. Role of itopride in minimizing post prandial glucose excursion in type 2 diabetic patients. J Med Sci. 2014;22:73–5.
- Camilleri M, Grover M, Farrugia G. What are the important subsets of gastroparesis? Neurogastroenterol Motil. 2012;24:597–603.
- Kassander BP, Hampshire N. Asymptomatic Gastric Retention in Diabetics (Gastroparesis Diabeticorum). Ann Intern Med. 1958;48:797–812.
- Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Szarka LA, et al. The Incidence, Prevalence, and Outcomes of Patients With Gastroparesis in Olmsted County, Minnesota, From 1996 to 2006. Gastroenterology. 2009;136:1225–33.
- Patrick A, Epstein O. Review article: Gastroparesis. Aliment Pharmacol Ther. 2008;27:724–40.
- Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: Diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589–91.
- Bielefeldt K. Regional Differences in Healthcare Delivery for Gastroparesis. Dig Dis Sci. 2013;58:2789–98.
- Waseem S, Moshiree B, Draganov P V. Gastroparesis: Current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25–37.
- Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9–19.