|
Jitendra Mistry1, Prashant Buch2, Udayan Kacchi3, Rakesh Vaidya4 1Surgical Gastroenterology, Mission Gastrocare, Vadodara, Gujarat, India. 2Hepatology, Liver Clinic, Vadodara, Gujarat, India. 3Pathology, Udayan laboratory, Vadodara, Gujarat, India. 4Pathology, Bhailal Amin Hospital, Vadodara, Gujarat, India.
Corresponding Author:
Dr Jitendra Mistry Email: jitlap@gmail.com
48uep6bbphidcol2|ID 48uep6bbphidvals|2986 48uep6bbph|2000F98CTab_Articles|Fulltext Solid pseudopapillary neoplasm of pancreas (SPNP) is considered a rare tumour of pancreas1. The incidence is increasing, possibly due to an increase in the use of cross-sectional imaging and immunohistochemistry. It is most frequent in young females2, usually occurs in the body-tail region of pancreas, compared to the head3,4 and carries an excellent prognosis with surgical resection. We present two cases of SPNP.
Case 1
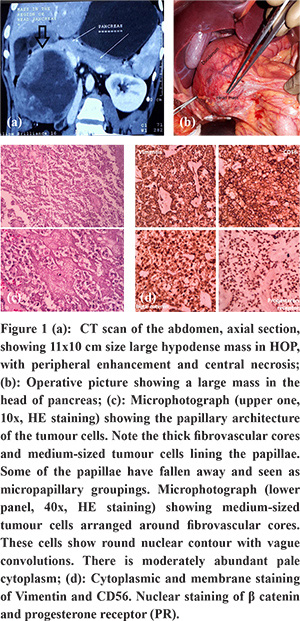
A 22 year-old-female patient presented with dull right upper abdomen pain for 1 year. She did not have jaundice, anorexia or weight loss. There was a palpable lump in the right hypochondrium. USG abdomen was suggestive of a large mass in the head of pancreas. Blood investigations, including liver function tests and pancreatic tumour markers, were within normal limits. CT scan abdomen with pancreatic protocol showed 11x10 cm size large hypodense mass in the pancreatic head, with peripheral enhancement and central necrosis (Figure 1a). The mass displaced the duodenum, bile duct and portal vein; and was touching the liver, right kidney and inferior vena cava. Based on clinical and imaging features, SPNP was suspected. As the patient was symptomatic, and the mass was resectable, the patient was offered pancreatoduodenectomy instead of endoscopic ultrasound (EUS) guided fine needle aspiration cytology (FNAC). She underwent classical Whipple’s procedure (Figure 1b). Postoperatively she had chyleinthe subhepatic drain, which was then left in situ for two weeks. She recovered well. Histopathological examination revealed features ofSPNP (Figure 1c). IHC examination confirmed the diagnosis with tumour cells expression for ß-catenin, CD-56, vimentin and progesterone receptor (PR) (Figure 2d). We followed her up with cross-sectional imaging every 6 months. At 21 months of follow up, she did well without any recurrence.
Case 2
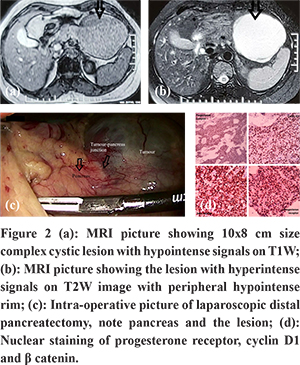
A 39 year-old-female patient developed fever and abdominal discomfort two weeks prior to presentation. USG abdomen revealed a large cystic mass in distal body-tail of the pancreas. Further evaluation with contrast-enhanced CT scan showed a large 10x8 cm size cystic lesion with enhancing wall and internal septation in the distal body of pancreas. To further clear the diagnostic dilemma, she underwent MRI abdomen (Figure 2a and b), which showed, 10x8 cm size complex cystic lesion with a peripheral hypointense rim on T2W images. The lesion revealed hypointense signals on T1W images and hyperintense signals on T2W images. Given the possibility of complex cystic neoplasm of pancreas, she underwent laparoscopic distal pancreatectomy (Figure 2c) and discharged on postoperative day 4. Histopathology examination revealedfeatures of SPNP, confirmed on IHC with tumour cells expressed ß-catenin, Cyclin D1 and PR (Figure 2d). At seven months of follow up, she was doing well without recurrence.
Discussion
SPNP is considered a rare neoplasm of the pancreas; it consists of 2-3% of pancreatic neoplasms1. Although a recent increase in the incidence of SPNP is apparent, it may be due to the rise in the detection rate because of the increased use of cross-sectional imaging and IHC. It is more common in young females, as were both of our patients; only 10% of SPNP occurs in males2. The origin of the tumour is debatable; and is often classified as an indolent tumour but potentially malignant. SPNP can occur anywhere in the pancreas, but it is more common in body and tail of pancreas compared to the head3,4 one of our cases had a tumour in the head while other had in the body-tail region of the pancreas. SPNP are either detected incidentally or present with a variety of non-specific symptoms like abdominal pain, nausea, vomiting and rarely jaundice. Both of our caseswere symptomatic with abdominal pain, without jaundice despite the involvement of head of pancreas by a large tumour in case 1. Among cross-sectional imaging, MRI is the preferred choice over a CT scan5. Characteristic findings on imaging include an encapsulated mass with solid and cystic components, there may be calcification or haemorrhage within the tumour. The solid areas enhance on pancreatic phase; slightly lesser than usual pancreatic parenchyma, the enhancement increases in delayed venous phase6. SPNP is usually oval in shape in females while lobulated in males7. EUS guided FNAC along with IHC helps in making preoperative diagnosis8 where there is a dilemma, although in many cases; clinical and imaging information provides enough evidence to go ahead for surgery. The overall prognosis of SPNP is excellent9. These tumours are indolent, with low malignant potential; up to 15% develop metastasis4. Young age, male sex, size more than 5 cm size, capsular invasion and high mitotic rate suggest possible malignant behaviour10,11. Surgical resection is the treatment of choice, parenchyma preservation with either enucleation or central pancreatectomy can be attemptedwhen feasible4. Spleen is preserved if there is no splenic vascular or splenic hilar involvement. Routine lymphadenectomy is not required as lymph node metastasis is rare12. Kim MJ et al. found a low recurrence rate with only 2 out of 106 patients having recurrence after a median follow up of 56.9 months13. Tang LH et al. reported a 97% five-year survival despite the presence of metastasis9. Role of adjuvant therapy in SPNP is unclear, while neoadjuvant treatment for down-staging of unresectable tumours using combination of gemcitabine and radiotherapy has been attempted14.
References - Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroentero 2006; 12(20): 3180–3185.
- Lanke G, Ali FS, Lee JH. Clinical update on the management of pseudopapillary tumour of pancreas. World J Gastrointest Endosc. 2018 Sep 16;10(9):145-155. doi: 10.4253/wjge.v10.i9.145. PMID: 30283597; PMCID: PMC6162250.
- Reindl BA, Lynch DW, Jassim AD. Aggressive variant of a solid pseudopapillary neoplasm: a case report and literature review. Arch Pathol Lab Med 2014; 138:974–978. [PMID: 24978926]
- Pantiora E, Vezakis A, Kollia D, Karvouni E, Politi AN, Kontis EA, Polydorou A, Fragulidis GP. Solid Pseudopapillary Neoplasm of the Pancreas - A Report of Two Cases and a Short Review of the Current Literature. J Pancreas (Online) 2018 Sep 28; 19(5): 251-257
- Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumour of pancreas: emphasis on magnetic resonance imaging findings. World J Gastroenterol 2007; 13: 1811-1815 [PMID: 17465471 DOI: 10.3748/wjg.v13.i12.1811]
- Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH, Lee JY, Han JK, Choi BI. Small (<or=3 cm) solid pseudopapillary tumours of the pancreas at multiphasic multidetector CT. Radiology 2010; 257: 97-106 [PMID: 20663966 DOI: 10.1148/radiol.10092089]
- Park MJ, Lee JH, Kim JK, Kim YC, Park MS, Yu JS, Kim YB, Lee D. Multidetector CT imaging features of solid pseudopapillary tumours of the pancreas in male patients: distinctive imaging features with female patients. Br J Radiol 2014; 87: 20130513 [PMID: 24472726 DOI: 10.1259/bjr.20130513]
- Singh A, Mohan G, Chaturvedi S, Sarangi L. Solid pseudopapillary tumour of pancreas: A lesser known entity-diagnosis and pitfalls: A case report. J Cytol 2016; 33:229-232. [PMID: 28028341]
- Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumours of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases.
- Ugras N, Yerci Ö, Coskun SK, Ocakoglu G, Sarkut P, Dündar HZ. Retrospective analysis of clinicopathological features of solid pseudopapillary neoplasm of the pancreas. Kaohsiung J Med Sci 2016; 32:356-361. [PMID: 27450024]
- Lubezky N, Papoulas M, Lessing Y, Gitstein G, Brazowski E, Nachmany I, et al. Solid pseudopapillary neoplasm of the pancreas: Management and long-term outcome. Eur J Surg Oncol 2017; 43:1056-1060. [PMID: 28238521]
- Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg 2006; 93: 733-737 [PMID: 16609955 DOI: 10.1002/bjs.5334]
- Kim MJ, Choi DW, Choi SH, Heo JS, Sung JY. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg 2014; 101: 1266-1271 [PMID: 25052300 DOI: 10.1002/bjs.9577]
- Bansal A, Kaushal V, Kapoor R. Solid pseudopapillary tumours of the pancreas: Is there a role for adjuvant treatment? Saudi Surg J 2016; 4:47-51
|