48uep6bbphidcol2|ID
48uep6bbphidvals|2979
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Gall bladder cancer (GBC) is a disease with a dismal prognosis.1 If possible, complete surgical resection is the only treatment that offers hope for long term survival. However, almost two-thirds of patients who have undergone a potentially curative resection eventually succumb to recurrent disease, and the recurrence usually occurs at a distant site.2 Adjuvant strategies [chemotherapy (CT), chemoradiotherapy (CRT)] have been tried to improve the outcomes but the results are not consistent.3,4 Neoadjuvant therapy may provide benefits of CRT/CT delivered in the preoperative setting with the hope of increased resectability and early control of micro-metastases. It may also help screen the biologically aggressive tumours that progress while on therapy and thereby spare the morbidity of futile aggressive surgical resections. Although CT and CRT have shown some benefit in the palliative setting,5-8 there are only limited data on the role of neoadjuvant therapy in GBC. We conducted this study to assess the feasibility of two different regimens of neoadjuvant chemoradiotherapy (NACRT) in locally advanced GBC.
Methods
This study was done at the All India Institute of Medical Sciences, New Delhi. Patient recruitment was done between November 2011 and March 2013. The trial was approved by the Institute Ethics committee and registered with Clinical Trials Registry–India (www.ctri.gov.in; CTRI/2011/12/002264). The follow-up data was updated till Jan 2019.
Inclusion & exclusion criteria
All patients with GBC were assessed for inclusion into the study if they met the following criteria
1. Age between 18 and 65 years
2. Cytological proof of GBC
3. Resectable (T3–T4, N0–1, M0 as per AJCC 7th edition) disease on a dual-phase Computed Tomography (DPCT)
4. Not received any prior treatment for GBC
The patients were excluded from the study if they had
1. Poor performance status (Eastern Cooperative Oncology Group [ECOG] >2)
2. Any contraindication for giving chemotherapy and/or radiotherapy
3. Evidence of peritoneal dissemination, non-contiguous liver metastasis, and/or involvement of the main portal vein or main hepatic artery.
All patients underwent a staging laparoscopy prior to inclusion in the study to exclude any evidence of dissemination.
After the initial evaluation, all patients who had jaundice underwent pre-therapy biliary drainage to decrease the serum bilirubin to =3 mg/dl. All enrolled patients, after obtaining informed consent, were alternately allocated to receive either sequential NACRT (sNACRT; Figure 1A) or concurrent NACRT (cNACRT; Figure 1B)
The radiotherapy volume and portals were decided based on the pre-treatment CT scan. The radiotherapy technique consisted of 3-dimensional conformal radiation therapy (3D-CRT) planning and dose delivery either on cobalt or linear accelerator machines. The 3D-CRT computerised dose planning was carried out and approved before the dose delivery. Whole liver and duodenum dose was limited to <30 Gy.
All patients were monitored for toxicity, and the National Cancer Institute common terminology criteria for adverse events (CTCAE) v.4 (Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf) was used for defining toxicity.
Patients were reassessed clinically and had a repeat dual-phase CT scan done 3-4 weeks after completion of NACRT. Radiological assessment was done using the RECIST (response evaluation criteria in solid tumours) criteria version 1.1.9 Surgical resection was offered to all patients who had resectable disease on the reassessment imaging.
The primary endpoints were the ability to achieve surgical resection and radiological response. The secondary endpoints were toxicity, pathological response, and overall survival. The pathological response was graded as per Mandard’s grading.10
The patients were followed up three-monthly for the first year and six-monthly after that. At each follow-up visit, a detailed clinical examination was done. In addition, an ultrasound of the abdomen, liver function tests, haemogram, and chest X-ray were done. At one year postoperatively or earlier, if indicated, a CECT scan of the abdomen was done to rule out loco-regional recurrence.
Statistical analysis
All data were entered in Microsoft Excel 2007. The analysis was done using SPSS software version 11.5. Categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables were analyzed using Student’s t-test. A p-value of <0.05 was considered significant. Survival analysis was done using Kaplan–Meier survival curve, and the probabilities were compared using the log-rank test. The overall survival (OS) was calculated as the time from the date of start of NACRT to death or date last known alive.
Results
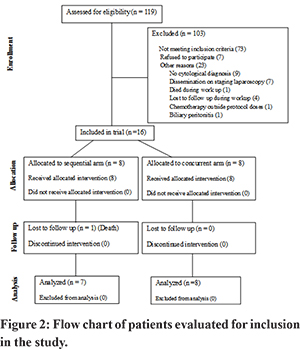
Between November 2011 and March 2013, 119 consecutive patients of suspected gallbladder cancer on ultrasound or CT scan were evaluated for inclusion. Of these, 103 patients were excluded for various reasons, and 16 were finally included in the study (Figure 2). Eight patients each were allocated to sNACRT and cNACRT arms. The baseline characteristics of the patients in the two arms have been provided in Table 1.

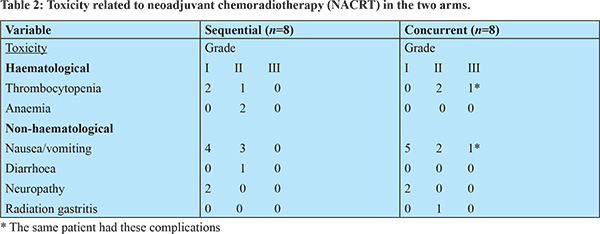
The mean duration between allocation to the treatment arms and start of NACRT was 30.7±16.6 and 33.7±11.5 days in the sNACRT and cNACRT arms, respectively (p=0.597). Neoadjuvant therapy was well tolerated in both arms. The most common toxicity was nausea/vomiting, followed by thrombocytopenia (Table 2). There was no grade III/IV toxicity in the sNACRT arm, while only one patient developed grade III toxicity (vomiting and thrombocytopenia) in the cNACRT arm. There was no treatment-related death. One patient, each in the sNACRT and cNACRT arms, had a break in the therapy. In the sNACRT arm, the patient had stent block cholangitis, and therapy was resumed once the cholangitis had resolved. In the cNACRT arm, the patient had grade III vomiting and thrombocytopenia. This patient completed radiotherapy after a brief period of hospitalization, but the last two doses of chemotherapy had to be omitted.
One patient in the sNACRT arm died of severe cholangitis due to a stent blockade before the response could be assessed. In the sNACRT arm, one patient had a complete radiological response (CR), two had partial responses (PR), and four had progressive disease (PD) while in cNACRT arm there was one CR, six PR and one stable disease (SD) with none having disease progression (PD). Thus, the overall response rate in the sNACRT arm was 3/7 (43%), while in the cNACRT arm, it was 8/8 (100%; p=0.025).
Six patients in the sNACRT arm could not be offered surgical resection. Four patients had progressive disease, one died of cholangitis, and another one was lost to follow-up. Two patients in the cNACRT arm did not undergo resection. One of these was admitted for surgery but developed cholangitis due to choledocholithiasis for which he underwent ERC/stenting. Though his cholangitis improved, his performance status deteriorated and hence was not offered surgery. The other patient was not offered surgery because the tumour was infiltrating the main portal vein. In view of significant response to NACRT based on RECIST and a PET-CT scan, he was given six further cycles of chemotherapy following which he developed a complete radiological response. Surgery was not offered as he had portal vein thrombosis. He did well and survived for 29 months.
Surgery could be offered to two patients in the sNACRT arm and six patients in the cNACRT arm. Among the operated patients, the duration between allocation to a treatment arm and surgery was around 150 days in the sNACRT arm while it was around 100 days in the cNACRT arm. This difference was because the sequential group had a longer NACRT protocol. The interval between completion of NACRT and surgery was around six weeks in both the arms. The operative details and outcomes of the patients who underwent surgery have been provided in Table 3. A specific intraoperative finding attributable to NACRT was fibrosis in the hepatoduodenal ligament (HDL). Dense fibrosis was seen in two patients in each arm. There was difficulty in the clearance of the HDL during surgery in patients who had dense fibrosis.
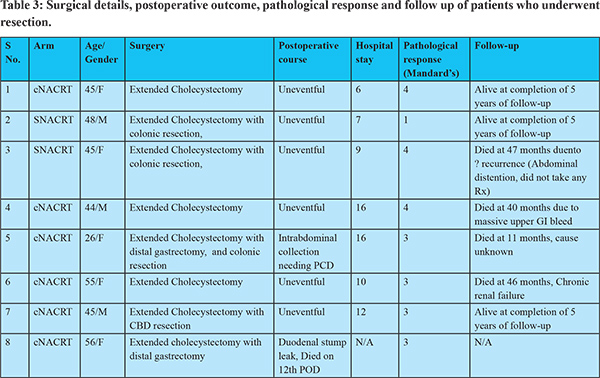
Of the eight patients who underwent surgical resection, two (both in cNACRT arm) developed significant complications. One patient developed severe sepsis due to a duodenal stump leak and subsequently succumbed. Another patient developed intra-abdominal collection and required percutaneous drainage and antibiotics.
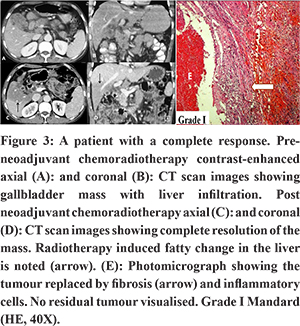
In the sNACRT arm, the pathological response (Mandard’s grading) was grade I (Figure 3) in 1 and grade IV in 1 patient. In cNACRT arm, four patients had grade III, and two patients had grade IV response. All patients had R0 resections. The surgical specimens were also examined for changes due to chemotherapy (steatosis, sinusoidal dilatation, hemorrhage, and nodular hyperplasia) and radiotherapy (veno-occlusion and peri-sinusoidal hepatocyte atrophy). Most patients had mild changes; however, one patient in sNACRT arm with complete pathological response had grade 3 sinusoidal dilatation.
All surviving patients have completed five years of follow up. The median overall survival was 8.6 months (95% CI 5.3–46.3) and 28.8 months (95% CI 6.6–45.1) in sNACRT and cNACRT arms, respectively (p=0.331). The survival was significantly higher in patients who underwent surgery as compared to those who did not [45.1 months (95% CI 10.2-46.3) vs. 6.6 months (95% CI 5.3-9.4); p=0.002]. In the sNACRT arm, all of the six patients who did not undergo surgery died while one of the two patients who underwent surgery is still alive. In the cNACRT arm, both the patients who were not operated have died, and among the six who were operated, two are still alive.
Discussion
This study was done to assess the safety and efficacy of NACRT protocols in patients with LA-GBC. We could include only 16 of the 119 eligible patients, and the rest had to be excluded mainly in view of advanced disease. This reflects the overall dismal outcome of patients with GBC. Other studies have also shown that only 10%-31% of patients presenting with GBC are suitable for surgery.11-13
Toxicity and compliance are major issues against NACRT. In our study, the NACRT regimens were well tolerated as only one patient had Grade III toxicity. The compliance was also good, with no patient discontinuing treatment. The toxicity profile was similar in both arms. De Arextabala et al.14 reported mainly hematological toxicity (20/23) in their patients. In the phase III trial from AIIMS, New Delhi evaluating GEMOX in GBC, the reported grade 3 or 4 toxicities were vomiting (7.7%), myelosuppression (38.5%), neurotoxicity (11%), and transaminitis (15%).8 Toxicity rates can be extrapolated from pancreatic cancers where gemcitabine-based NACRT has been used extensively. In a recent meta-analysis, grade 3/4 toxicity was estimated at 29.4%.15
The radiological response rates were better in the cNACRT arm (100%) as compared to 43% in the sNACRT arm. The previously reported response rates achieved in GBC in the unresectable setting using gemcitabine/gemcitabine and cisplatin have been under 40% except for the trial by Sharma et al.8 that reported a response rate of around 70%.7,8
The mean time to the allocation of NACRT arm to surgery was around 22 and 14 weeks in sNACRT and cNACRT arms, respectively, which included a period of preoperative biliary drainage in jaundiced patients. Patients were operated after a mean of 40.5 and 37.6 days after completion of chemoradiotherapy in sNACRT and cNACRT arms. The ideal lag period between completion of NACRT and surgery remains undecided in this subset of patients. Of 11 patients with response, 8 underwent resection (2 out of 3 in sNACRT arm and 6 out of 8 in cNACRT arm).
None of the patients taken up for surgery had metastasis or unresectable disease. Thus, neoadjuvant treatment might have helped in selecting patients with better biology (who responded to therapy), thereby avoiding a non-therapeutic laparotomy. A specific NACRT related problem faced during surgery was RT-induced fibrosis in the HDL; abbreviation already expanded earlier in some patients, which led to difficulty in identifying the portal structures and could have compromised complete lymph nodal clearance. However, only one patient presented later with lymph nodal recurrence in the retro-pancreatic region at nine months, which may be attributable to inadequate clearance of the HDL.
The pathological response is the most definitive evidence of response to NACRT. Of the operated patients, one had a pCR, and the rest had a pPR. With no trials of NACRT in GBC, much of the data has to be extrapolated from the metastatic settings, which have shown occasional pCR in a few patients who could be operated.16 Most importantly, all operated patients in our study had an R0 resection. It is well known that the most significant factor determining long term survival is whether the patient had an R0 resection.
The surgical specimens were analysed for the presence and severity of NACRT induced changes in the liver. This is of concern to hepatobiliary surgeons as radical surgery for GBC may involve a variable amount of liver resection. Pathological analysis revealed only mild to moderate changes, and the only patient to have grade III sinusoidal dilatation was the one with pCR.
At a median follow-up of 60.1 months for surviving patients, the median overall survival was higher in the cNACRT arm; 28.8 vs. 8.6 months though this did not reach statistical significance, possibly because of a small sample size. The mean survival in all patients who underwent surgery was significantly higher; 45.1 vs. 6.6 months in patients who did not undergo resection (p=0.002). However, the numbers are too small to make a definitive conclusion about survival outcomes.
Since the start of our study, there has been one prospective study from another tertiary care centre in India that looked at NACRT for locally advanced GBC.17 Over a period of five years, they could enroll 28 patients of stage III GBC. Diagnostic laparoscopy was not a part of their protocol, and they used a higher dose of radiotherapy (57 Gy) with gemcitabine monotherapy. They reported 11% grade III toxicity with a 71% response rate. Only half of these patients could undergo R0 resection. Postoperative bile leak rates were high (43%), possibly linked to higher radiotherapy doses. At a median follow-up of 37 months for surviving patients, the OS was 20 months and 35 months for those who underwent an R0 resection.
Conclusions
This pilot study aimed to assess the feasibility, safety, and efficacy of NACRT in resectable LA-GBC in terms of tumour downstaging and its effect on overall survival. Although safe and efficacious in response, the NACRT protocol could be offered to a select group of patients with locally advanced gallbladder cancer (16/119; 13.5% who presented to us in the outpatient clinic). The delay in surgery was between 14 and 22 weeks for concurrent and sequential NACRT regimens, respectively, and increased further when patients were jaundiced. Concurrent NACRT had better results in response rate, ability to achieve surgical resection, and overall survival. A larger study will provide a better assessment of the impact of these regimens on disease-free and overall survival.
References
- Ertel AE, Bentrem D, Abbott DE. Gall Bladder Cancer. Cancer Treat Res. 2016; 168: 101–120.
- Masior L, Krasnodebski M, Kobryn K, Grat M, Krawczyk M. Surgical treatment of gall-bladder cancer. Pol Przegl Chir. 2015 Jun; 87: 324–330.
- Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. The Oncologist. 2010; 15: 168–181.
- Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol Off J Am Soc Clin Oncol. 2012 Jun 1; 30: 1934–1940.
- Gallardo JO, Rubio B, Fodor M, Orlandi L, Yáñez M, Gamargo C, et al. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2001 Oct; 12: 1403–1406.
- Teufel A, Lehnert T, Stremmel W, Rudi J. Chemotherapy with gemcitabine in patients with advanced gallbladder carcinoma. Z Gastroenterol. 2000 Nov; 38: 909–912.
- Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004 Apr 19; 90: 1516–1520.
- Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol Off J Am Soc Clin Oncol. 2010 Oct 20; 28: 4581–4586.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990. 2009 Jan; 45: 228–247.
- Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994 Jun 1; 73: 2680–2686.
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010 Apr 8; 362: 1273–1281.
- Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992 Sep 15; 70: 1493–1497.
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000 Oct; 232: 557–569.
- de Aretxabala X, Losada H, Mora J, Roa I, Burgos L, Yáñez E, et al. [Neoadjuvant chemoradiotherapy in gallbladder cancer]. Rev Med Chil. 2004 Jan; 132: 51–57.
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010 Apr 20; 7: e1000267.
- Sharma A, Mohanti B, Raina V, Shukla N, Pal S, Dwary A, et al. A phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother Pharmacol. 2010 Feb; 65: 497–502.
- Engineer R, Goel M, Chopra S, Patil P, Purandare N, Rangarajan V, et al. Neoadjuvant Chemoradiation Followed by Surgery for Locally Advanced Gallbladder Cancers: A New Paradigm. Ann Surg Oncol. 2016; 23: 3009–3015.