48uep6bbphidcol2|ID
48uep6bbphidvals|2975
48uep6bbph|2000F98CTab_Articles|Fulltext
Mucormycosis (zygomycosis) is a mould infection caused by a group of ubiquitous fungi (Mucorales). Rhizopus oryzae is the most common cause of mucormycosis1. The risk factors for the development of invasive mucormycosis are diabetes mellitus, particularly with ketoacidosis, corticosteroid use, neutropenic states in organ/stem cell transplantation or hematologic malignancies, malnourished states in renal failure, low birth infants, human immunodeficiency virus (HIV) infection and states of iron overload or treatment with desferoxamine. Nosocomial outbreaks may occur by transmission through contaminated bandages, tongue depressors and intravenous catheters1.
We present a case of invasive intestinal mucormycosis presenting with pulmonary symptoms in an elderly diabetic woman.
Case Report
A 75-year-old female presented to us with complaints of shortness of breath for the last 10 days and cough with purulent expectoration for one week. She also had severe right lower quadrant abdominal pain for the last two days. Her medical history revealed that she had hypertension for the past 15 years, uncontrolled diabetes for the last 10 years on oral hypoglycaemic agents glimepiride and metformin and chronic obstructive airway disease (COAD) treated with bronchodilators and inhalation steroids. She had been evaluated at an outside hospital for her current complaints with contrast enhanced computed tomography (CT) abdomen which revealed long segment enhancing circumferential wall thickening in the distal ileal loops, cecum and ascending colon. She was referred to us for further management. On admission, she was hypotensive with a blood pressure of 80/50 mm of Hg, and pulse rate of 120 beats/min. Systemic examination was normal except for bilateral pitting pedal oedema and right basal coarse crepitations. Laboratory examination showed haemoglobin of 14gm/dL, total leucocyte count of 22800/mm3 with neutrophils being 92%, and platelet count of 1.85 L/mm3. Serum creatinine was 0.85 mg/dL. Serum electrolytes were normal. Liver function tests were normal except for hypoalbuminemia (serum albumin: 3gm/dl). She had normal coagulation parameters.
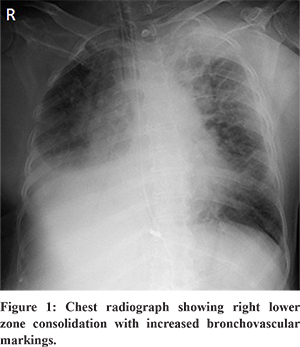
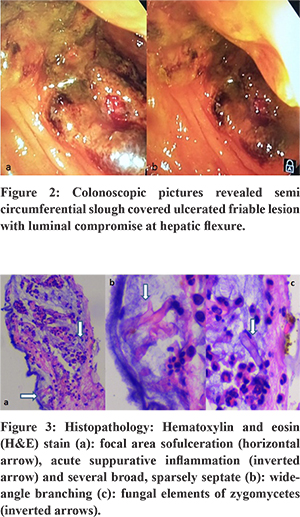
Her chest radiograph showed right lower lobe consolidation (Figure 1). Arterial blood gas analysis showed metabolic acidosis. She was intubated in view of tachypnea and hypoxemic respiratory failure. She was admitted in an intensive care unit and started on parenteral broad spectrum antibiotics, inotropes and fluid resuscitation. Bed side colonoscopy revealed a semi-circumferential slough-covered ulcerated friable lesion with luminal compromise at the hepatic flexure (Figure 2). The rest of the visualised colonic mucosa was normal. Multiple biopsies were taken from the abnormal mucosa to ascertain the aetiology. Histopathology showed focal areas of ulceration and acute suppurative inflammation. Several broad , sparsely septate , wide angle branching fungal elements were noted within the mucoid material and within the crypt lumen suggestive of mucormycosis (Figure 3). Microbiological culture and species subtyping was not done. She was started on intravenous amphotericin-B (5 mg/kg/day), but she succumbed to the illness after 5 days of treatment.
Discussion
Mucormycosis may be classified as rhino-cerebral, pulmonary, cutaneous, GI or disseminated disease. Rhino-cerebral mucormycosis is due to the inhalation of spores into the paranasal sinuses of susceptible individuals and is the most common clinical presentation. Gastrointestinal (GI) mucormycosis is rare and is acquired by the ingestion of pathogens in foods such as fermented milk, dried bread products, and fermented porridge2. The stomach is the most common site (58%), followed by the colon (32%). The ileum and the oesophagus are rare sites of involvement. Patients present with abdominal pain, hematemesis and necrotic ulcers that can lead to perforation peritonitis and shock with poor prognosis2.
The diagnosis of GI mucormycosis relies upon the identification of organisms on endoscopic biopsy and histopathology. Tissue culture often yields no growth. Mucormycosis hyphae are broad (5 to 15 micron diameter), irregularly branched, and have rare septations in contrast to Aspergillus septate hyphae which are narrower (2 to 5 micron diameter) with regular branches. Serum tests (1,3-beta-D-glucan assay and the Aspergillus galactomannan assay) can detect aspergillosis but not mucormycosis due to the lack of these cell wall components1. The presence of more than 10 pulmonary nodules, pleural effusion, concomitant sinusitis, reverse halo signs on CT chest and prior voriconazole prophylaxis is more suggestive of mucormycosis than aspergillosis3.
Tissue necrosis due to angioinvasion and vascular thrombosis by hyphae prevents tissue penetration of antifungal agents and surgical debridement becomes essential in treatment1.
Treatment of GI mucormycosis involves a combination of surgical debridement of involved tissues, antifungal therapy and elimination of predisposing factors for infection, such as hyperglycemia, metabolic acidosis, deferoxamine administration, immunosuppressive drugs, and neutropenia1. Intravenous amphotericin B is the drug of choice for initial therapy1. Posaconazole is used as step-down/salvage therapy for patients who have responded to /intolerant of amphotericin B1.
The duration of antifungal treatment is guided by the resolution of clinical and radiological features which may take 6 to 8 weeks. The recommended therapeutic drug level of posaconazole is a trough concentration of 1 µg/mL or higher4. Delayed presentation with inability to remove the infected intestinal segment and use of parenteral antifungal therapy alone in an immunocompromised patient might have led to fatality in our case. Therefore, a high degree of clinical suspicion, early diagnosis and prompt surgical debridement with anti -fungal therapy might improve survival.
References
- Thomas L, Tay SY, Howard D, FalhammarH.Mucormycosis in a 40-year-old woman with diabetic ketoacidosis.CMAJ. 2020;192(16):431-433.
- Agha FP, Lee HH, Boland CR, Bradley SF. Mucormycoma of the colon: early diagnosis and successful management. AJR Am J Roentgenol 1985; 145:739.
- Chamilos G, Marom EM, Lewis RE, et al. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis 2005; 41:60.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019;19:405–21.
- Quraishi AHM, Peshattiwar A, Umare G, Bannerji A. Perforation Peritonitis Secondary to Intestinal Mucormycosis in a Boy with Type I Diabetes Mellitus. J Indian Assoc Pediatr Surg. 2020;25:118-120.