48uep6bbphidcol2|ID
48uep6bbphidvals|2965
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
According to the Global Health Observatory data from the World Health Organization (WHO), the liver disease burden is huge with 22.2 deaths per 100,000 population attributed to cirrhosis1. It has been conservatively estimated that at least 20,000 people in India require a liver transplantation annually2. At present, only around 800 to 1000 liver transplantations are being performed annually.
The primary legislation related to organ donation and transplantation in India, Transplantation of Human Organs Act, was passed in 1994. Despite the law being in place, the deceased donation rate has seen little progress. As a consequence, the rate of DDLTs (Deceased Donor Liver Transplantations) have been very low, with more than80% of the transplants being LDLTs (Living donor liver transplantations)3.
At present, the organ allocation is facilitated by the state coordinating body which allocates donated livers on a rotational basis to licensed centers within the state.More than 200 centers across India are recognized by the government to perform liver transplantation4. Due to the low rate of organ donations, the number of DDLTs done in each center has been low and it has been impractical for each center to house a comprehensive transplantation team.
Our team at Manipal hospitals, has been working with a “Core-Satellites Model” to ensure maximum services with the available resources. The transplantation team was housed at one core center but performed transplantationsat two peripheral centres as well. The postoperative management of the transplanted patient at the peripheral centreswas carried outremotely using telemedicine modalities.This study was undertaken to evaluate the working of this model. The hypothesis was that patients managed atsatellite centres had outcomes equivalent to those managed in the core center by an in-house team.
Methods
Patients who underwent DDLTs at Manipal hospitals between November 2016 and January 2018 were included in the study. All patients studied were aged above 18 years and had undergone their first liver transplants. Patients receiving combined grafts were excluded. The grafts were procured from brain dead (DBD) donors. All patients received whole grafts.
The transplantations were undertaken at three hospitals. Manipal hospital (MHB), Bangalore in the state of Karnataka was considered the core hospital which housed the transplantation team. Manipal hospital (MHS), Salem in the state of Tamil Nadu and Manipal hospital (MHV), Vijayawada in the state of Andhra Pradesh were considered satellite units. These centres did not have any doctor or paramedical personnel with prior exposure to liver transplantation. MHS is 202 km from MHB and is connected to it by road with a travel time of approximately 3 hours and 30 minutes. MHV is 646 km from MHB and is connected by flight. There were 2 flights per day between Bangalore and Vijayawada with a flight time of 1 hour and 15 minutes (Figure 1).
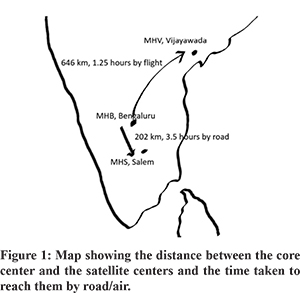
When information of a potential donor in a satellite hospital wasreceived, the team from the core hospital would go to the satellite hospital by road or by air. After transplant, one member of the core team would stay back in the satellite center for 3 to 4 days till the patient was rendered stable (extubated and tolerating oral diet). The management of the patient is then taken over by the local team of intensivists, surgeons and gastroenterologists. The reports are sent back to the core team by Whatsapp. The patient is viewed through Skype by the core team, which guides the local team in further management till discharge. In case of any complication that cannot be handled by the local team, either the patient is brought back to the core center or one member of the core team goes back and takes over the post-operative management.
The aim of the study was to compare the outcomes of patients transplanted in the resource-poor satellite centers when compared to the outcomes of patients transplanted in the core center.
The recipient variables that were observed were: age, sex, BMI, the MELD score and the presence of portal vein thrombosis. The donor characteristics observed in both groups were: age, sex, peak sodium levels, hemodynamic instability before donation, type of arterial anatomy of the graft and the estimated steatosis of the graft. The number of blood transfusions required, and the ischemia times were the other characteristics that were observed.
The following outcomes were studied: Post reperfusion syndrome (PRS), early allograft dysfunction (EAD), mortality within the first 30 days after the transplantation and post-operative trend in the production of serum hepatic enzymes such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The length of hospital stay, the need for transfer to the core center and need for extended stay by the core team member at the satellite center were also studied.
PRS was defined as a decrease in mean arterial pressure greater than 30% below the baseline value, lasting for at least 1 min, occurring during the first 5 min after reperfusion of the liver graft (unclamping of the portal vein). EAD was defined as a peak value of aminotransferases >2000 IU/mL during the first week or an international normalized ratio of =1.6 and/or bilirubin =10 mg/dL on day 7 after the surgery.
All analyses were performed using the statistical software package STATA v.15.0. Continuous variables were described by means and standard deviations and categorical variables by frequencies and percentages. Comparisons between groups IGL-1 and UW were performed by Student t-test, Pearson’s Chi- square test and the Fisher’s exact test as applicable. The non-parametric Mann-Whitney test was used to compare preoperativebilirubin levels, post-operative bilirubin, INR and enzyme levels, and blood transfusion requirements between the groups. For all analyses, a P value <0.05 was considered as statistically significant.
Results
Eighty patients underwent DDLTs in the study period.At the core centre, MHB, 31 (38.75%) transplants were carried out and 49 (61.25%) transplants were carried out in the satellite centres (26 in MHV and 23 in MHS). Recipient, donor and graft baseline characteristics according to each group are presented in table 1. The patients operated at the core center were found to be more elderly (55.10 ± 10.10 years) than those operated at the satellite centers (49.76 ± 10.95). There were no differences in terms of recipient’s sex, height, weight, BMI, presence of hypertension, presence of diabetes, preoperative bilirubin, preoperative INR, preoperative creatinine, the MELD score, the aetiology of the liver disease and the presence of HCC.
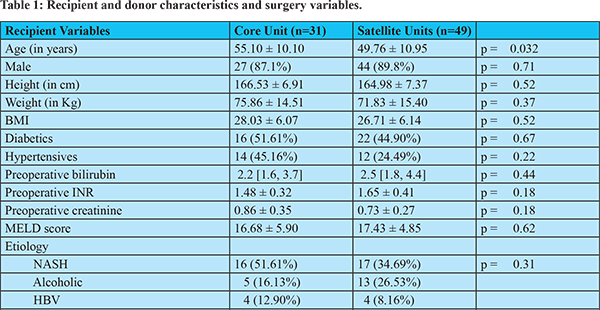
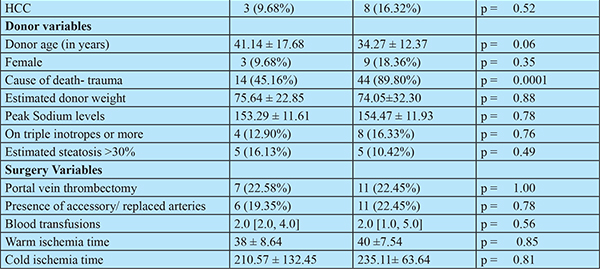
The donors in the core hospital were older than those in the satellite centres (mean age (41.14 ± 17.68 vs34.27 ± 12.37 years), though not significant statistically (p=0.06). The cause of death was trauma more often in the satellite centers than in the core center (89.80% vs 45.16%, p =0.0001). The other donor characteristics were comparable: sex, peak sodium levels, estimated weight of the donor, the number of donors on high (3 or more) inotropic support and the proportion of patients with more than 30% steatosis of the graft. The proportion of patients who underwent portal vein thrombectomy, the proportion of grafts which had accessory/replaced hepatic arteries, the ischemia times and the number of blood transfusions required were also comparable.
The outcome parameters are presented in table 2. The peak AST levelswere comparable between the 2 groups. The peak postoperative bilirubin levels were higher in the satellite centers (4.3 [2.5, 6.1] vs 2.3 [1.9, 4.3], p = 0.020) and the peak postoperative INR levels were higher in the core center (4.2 [3.1, 5.1] vs 2.2 [1.7, 2.7], p < 0.001). The incidence of PRSand 30 day- mortality were not found to be significantly different between the two groups. More patients operated in the core center had early graft dysfunction than those operated in the satellite centers (35.48% vs 10.20%, p = 0.006). The period of hospitalisation waslonger at the core center than in the satellite centers (20.59 ± 10.14 vs 15.96 ± 6.75 days p = 0.018).
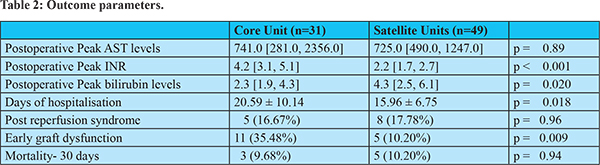
Table 3 lists the causes of death within 30 days after transplantation in the three centers and table 4 lists the instances wherein the patient needed to be shifted back or the primary transplant team had to stay/go back to attend to the patient to tackle untoward issues.
Discussion
Telemedicine is the use of telecommunication to deliver clinical health care from a distance. In the field of liver transplantation, telemedicine has been used in different scenarios5. Tele-radiological and tele-pathological evaluation of the liver graft has been explored to make decisions on the utility of the graft6. Telemedicine has been used to monitor and provide care for transplanted children from abroad7. It has been used to guide anaesthesia during liver transplantation from a remote location8. It has aided communication between transplant teams when a graft needed to be split for two recipients9. This is the first instance where telemedicine has been used to monitor and provide care for patients in the immediate post-transplant period.
Though deceased organ donation was sporadic initially in India, it has found impetus in the last decade with the rate of organ donation going up from 0.05 per million populations to 0.8 per million population in a span of few years10. The existing organ sharing networks allot the donated livers to the licensed liver transplantation hospitals in the state. The existing rules do not allow the transport of donor organs across state borders under routine circumstances. Due to the low volume of donation, many hospitals perform less than 5 transplants per year. These low volume hospitals have found it difficult to house a full-fledged liver transplantation team. Hospitals which perform liver transplants in higher numbers, most of them being LDLTs, are staffed sufficiently with a comprehensive team of doctors and paramedical personnel andsupport the smaller volume centres during DDLTs.
While surgeons travelling to retrieve donated organs is a common occurrence worldwide, surgeons travelling to perform the more complex recipient surgery is infrequent. We at Manipal hospitals, have done more DDLTs in the satellite centers than in the hospital that houses the surgeons. We have used commonly available telecommunication modalities like Whatsapp and Skype to manage liver transplantation patients at distant centers in the immediate postoperative period. As seen in table 4, initially, the patients were transplanted back to the core center after they were stable enough for transport. As experience grew, the patients were managed in the satellite centers till they were fit for discharge. A member of the core team has had to go back in a few instances to handle postoperative complications when needed.
Analysis of the outcomes of the patients managed thus were found to be similar to those managed by an in-house transplant team. On the contrary, patients operated in the peripheral centers were found to have lesser early graft dysfunction than those operated in the core center. This might bea reflection of the difference in thegrafts procured in the different centers. In the satellite centers, the grafts were donated by younger patients who became brain dead after involvement in a road traffic accident. In the core center, the donors were more elderly with associated comorbidities who became brain dead after a cerebrovascular accident. The recipients were also younger on an average in the satellite centers when compared to those operated in the core center. The length of hospital stay was lesser in the satellite centers, which might indicate the eagerness of the patient to go back to the core center at the earliest.
Our experience has shown that surgeons travelling distances to perform liver transplantations and the use of telemedicine to provide postoperative care, can provide effective therapy to liver transplant patients. This working model can potentially increase the number of transplantations done at hospitals that are not fully staffed with a transplant team. The success of organ transplantation in smaller cities can, in turn, potentially increase the motivation for organ donations in the same locality.
In conclusion, utilisation of telemedicine has been useful in delivering effective postoperative care for liver transplantation patients at centers that were distant from the hospital that housed the core liver transplant team.
References
- GHO | By category | Age-standardized death rates, liver cirrhosis (15+) - by country [Internet]. WHO. [cited 2018 May 18]. Available from: http://apps.who.int/gho/data/view.main.53420
- Soin AS, Kakodkar R. Living donor liver transplantation in India. Trop Gastroenterol Off J Dig Dis Found. 2007;28:96–8.
- Narasimhan G, Kota V, Rela M. Liver transplantation in India. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2016;22:1019–24.
- Nagral S, Nanavati A, Nagral A. Liver Transplantation in India: At the Crossroads. J Clin Exp Hepatol. 2015 ;5:329–40.
- Ertel AE, Kaiser T, Shah SA. Using Telehealth to Enable Patient-Centered Care for Liver Transplantation. JAMA Surg. 2015 ;150:674–5.
- Mammas CS, Geropoulos S, Kavantzas N, Chanjiioannou A, Doteiras C, Saatsakis G, et al. Telemedicine systems in organ transplantation: a feasibility and reliability study of the integrated teleradiological and tele-pathological evaluation of the cardiac graft. Stud Health Technol Inform. 2014;202:303–6.
- Song B, Schulze M, Goldschmidt I, Haux R, Baumann U, Marschollek M. Home monitoring and decision support for international liver transplant children. Stud Health Technol Inform. 2013;192:268–72.
- Fiadjoe J, Gurnaney H, Muralidhar K, Mohanty S, Kumar J, Viswanath R, et al. Telemedicine consultation and monitoring for pediatric liver transplant. Anesth Analg. 2009;108:1212–4.
- Bhati CS, Wigmore SJ, Reddy S, Mayer DA, Buckels JAC, Derek M, et al. Web-based image transmission: a novel approach to aid communication in split liver transplantation. Clin Transplant. 2010 ;24:98–103.
- Pandit RA. Brain death and organ donation in India. Indian J Anaesth. 2017 ;61:949–51.