48uep6bbphidcol2|ID
48uep6bbphidvals|2941
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
High-resolution esophageal manometry (HREM) is currently an essential investigative tool for evaluating esophageal symptoms such as dysphagia, gastroesophageal reflux disease (GERD), and non-cardiac chest pain. Chicago classification v3.0 is utilized to report HREM. It incorporates multiple objective parameters like distal latency (DL), contractile front velocity (CFV), integrated relaxation pressure (IRP) and distal contractile integral (DCI).1
For the ease of interpretation of HREM, the esophagus is divided into three segments. S1 segment or the Transition zone (TZ)is a low-pressure zone straddled between the striated and the smooth muscle of the esophagus. S2 and S3 segments lie in the body of the esophagus, the latter merging with the lower esophageal sphincter (LES). Breaks up to 2 cm in both these segments areconsidered normal. For effective uninterrupted bolus transport across S1 through to S2 and S3, the contractile waves in striated and smooth muscle exhibit a smooth spatiotemporal coordination.2,3
In India, most centers use water perfused HREM. A large subset of patients are referred to these centers for evaluation of GERD and peristaltic abnormality before anti-reflux surgery. The presence of large peristaltic breaks in these patients often poses a clinical dilemma regarding further management. Although the present Chicago classification does not discuss breaks, we believe that these are relevant to our clinical practice and are likely to impact decision making. To address some of these issues, we prospectively assessed the HREM findings in symptomatic GERD patients to study the prevalence and significance of large segmental breaks.
Materials and Methods
The study was conducted at the GI Motility Unit at Gleneagles Global Health City, Chennai, between June 2012 and May 2017. Cases were enrolled using non-probability convenience sampling. Patients with symptoms of GERD referred for manometry or 24 hour pH testing were included for the study. Baseline patient information included age, gender,BMI, diet recall, and upper endoscopy report.
Exclusion criteria: Post fundoplication, scleroderma
The details of study recruitment and analysis are shown in Figure 1.
Manometry protocol: Medications likely to affect the smooth muscle contraction or LES relaxation such as prokinetics and anticholinergics were discontinued 14 days prior to the recording. Informed consent was obtained. HREM was recorded using 5 mL water for 10 swallows in the supine posture and a 16 channel water perfusion system (Ready Stock, Australia), and reported according to Chicago Classification v 3.04 using Trace 1.3.3 software (Hebbard, Australia). The median DL, DCI, and IRP were estimated. Breaks in S1, S2, and S3 segments were categorized as less than 2 cm (considered normal), 2 to 5 cm, and more than 5 cm.
Breaks in proximal and distal segments (Figure 2a) were compared for all swallows in patients with normal motility (Group I) and minor peristaltic abnormality (Group II).
Multiple rapid swallows (MRS) (Figure 2b) were performed toassess the esophageal peristaltic reserve. The process involved a series of single 5 swallows with 2?mLwater given at 10-second intervals. Abnormal MRS was defined when the post-MRS contraction was weak and was classified as Type IEM-Awhen DCI ratio between average 10 wet swallows and post-MRS contraction was <1 and type IEM-B when post-MRS contraction DCI was <450?mm Hg-s-cm.5
Patients in group I and II were compared for demography, dietary factors, and endoscopic grading -Los Angeles (LA) grades of esophagitis- to study the determinants of abnormal peristalsis.
Statistical analysis
For descriptive analysis of quantitative variables, mean and standard deviation, andfor categorical variables, frequency and proportion were applied. Independent student’s t-testand Chi-square test were used. Logistic regression was performed to determine cut off values for the significant factors, and sensitivity, specificity, relative risk, positive and negative predictive values were calculated. Ap-value <0.05 was considered as statistically significant.
Results
One hundred and six patients were referred for HREM. The mean age was 42.6+15.4 years, and BMI was 25.93 kg perm2. Seventy two (72) patients had normal esophageal motility (Group I), and 30 had minor peristaltic abnormality (Group II). Twenty one (70%) and 9 (30%) patients in the latter group had ineffective esophageal motility and fragmented peristalsis, respectively.
Age, gender distribution, and endoscopic parameters were comparable in the two groups. BMI was higher in Group II, and in both groups, the median BMI was > 25 kg per sq.m. Dietary factors like fried and spicy food were more frequent in group I (Table 1).
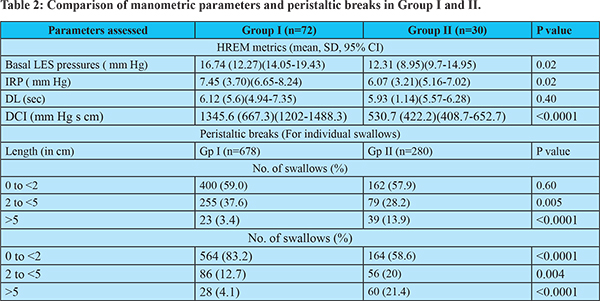
On analyzing the HREM, the mean basal LES pressure, IRP and DCI were lower in Group II. Also, a significant proportion of swallows in patients in Group II had breaks greater than 5 cm in S1 and more than 2 cm in S2 and S3. Seventeen of the 21 patients with ineffective esophageal motility in group II had MRS, 7 of whom had a poor peristaltic reserve (41.2%); 3 had Type IEM-A, and 4 had Type IEM-B. (Table 2)
To further study the combined effect of the low LES pressure and high BMI, on ineffective motility, we obtained a cut off value for both these parameters using linear regression. The basal LES pressure and BMI cut off were 12.1 mm Hg and 26.1 kg per m2, respectively. The odds ratio of having a minor peristaltic disorder was 3.2 times (95% CI 1.4-4.1, p= 0.001) with the combination of these two factors. Also the sensitivity, specificity, positive predictive value and negative predictive value for the 2 combined determinants were 60% (CI 40.6-77.4%), 77.8%(CI 66.4-86.7%), 52.3% (CI 40-65.4%) and 82.35% (74.7-88.4%) respectively.
Discussion
Majority of GERD patients from southern India had normal esophageal motility. Even in those with a minor peristaltic abnormality, the peristaltic reserve was good. This observation is similar to previous studies from India and abroad.6,7
Esophageal peristaltic breaks of > 5 cms size were frequent in group II in both the proximal (S1) and distal segments S2 and S3, which correlated well with lower mean DCI in these patients. Peristaltic breaks are known to impact esophageal clearance significantly. Ghosh et al8 noted that bolus retention in the TZ, i.e., S1, results in lower muscle squeeze in this segment. Consequently, there is a wide spatial separation between the upper and lower contraction waves, resulting in ineffective clearance. Pohl et al9 conceptualized that a time delay between the proximal and distal esophageal contraction waves may be significant in GERD symptoms such as dysphagia. In our study, we observed that larger distal defects, rather than proximal breaks, had a more noticeable impact on the occurrence of minor peristaltic abnormalities. Roman et al., reported that correlation of incomplete bolus transit with large breaks (>5 cm) was 100% and 16% with small breaks (2 to 5 cm).10 Recent studies have shown that proximal breaks too correlate with ineffective bolus transit.11
Apart from classifying various esophageal motor abnormalities, there has recently been a keen interest in stratifying patients with ineffective esophageal motility (IEM) with provocative tests like multiple rapid swallows (MRS). Repetitive and rapid swallows result in inhibition of the progression of peristalsis by a subsequent swallow, after which a high-amplitude peristaltic wave propagates along the esophagus.12,13 MRS testing may have implications while planning anti-reflux surgery for GERD.14
For the secondary endpoint, we looked into other factors that were responsible for the abnormal HREM findings. These patients had significantly lower basal LES pressure and higher BMI compared to those with normal HREM. A combination of these factors (basal LES pressure <12.1mm Hg and BMI >26.1kg per m2) had good sensitivity, specificity, and negative predictive value in the occurrence of peristaltic abnormalities. Dietary factors like fried food and spicy food were significantly higher in Group I than group II, but these did not affect the esophageal motility. These patients are likely to non-erosive reflux disease or hypersensitive esophagus. The majority of the symptomatic GERD cases had normal endoscopy (70, 68.6%), while the remaining had low-grade esophagitis ( LA A and B). These observations concur with reports from India which show that majority of Indian patients with GERD have low grade esopahgitis.15
The present study has a few limitations, including that the diagnosis of GERD was not confirmed with 24 hr pH monitoring or impedance testing. Whether the abnormal distal contractile element is the primary defect in GERD or a result of long-standing reflux is debatable and needs to be explored.
References
- Bredenoord MF AJ, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJPM. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. NeurogastroenterolMotil. 2012; 24:57–65.
- Ghosh SK, Janiak P, Schwizer W, Hebbard GS, Brasseur JG. Physiology of the esophageal pressure transition zone: separate contraction waves above and below. Am J Physiol. 2006; 290: G568–G576.
- Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol. 2006;290: G988–G997.
- International High Resolution Manometry Working Group. The Chicago Classification of Esophageal Motility Disorders, v3.0. Neurogastroenterology and motility?: the official journal of the European Gastrointestinal Motility Society. 2015;27:160-174.
- Min YW, Shin I, Son HJ, Rhee P-L. Multiple Rapid Swallow Maneuver Enhances the Clinical Utility of High-Resolution Manometry in Patients Showing Ineffective Esophageal Motility. Mayr. J, ed. Medicine. 2015;94(40): e1669
- Jain M, Baijal R. Characteristics of lower esophageal sphincter function and esophageal motility noted on High Resolution Esophageal Manometry in patients with reflux disease. Global Journal for Research Analysis. 2017;6:287-288.
- Ho SC, Chang CS, Wu CY, Chen GH. Ineffective esophageal motility is a primary motility disorder in gastroesophageal reflux disease. Dig Dis Sci. 2002;47:652–6
- Ghosh SK, Janiak P, Fox M, Schwizer W, Hebbard GS, Brasseur JG. Physiology of the oesophageal transition zone in the presence of chronic bolus retention: studies using concurrent high resolution manometry and digital fluoroscopy. NeurogastroenterolMotil. 2008; 20: 750–759.
- Pohl D, Ribolsi M, Savarino E, Fruhauf H, Fried M, Castell DO, et al. Characteristics of the esophageal low-pressure zone in healthy volunteers and patients with esophageal symptoms: assessment by high-resolution manometry. Am J Gastroenterol. 2008; 103: 2544–2549.
- Roman S, Lin Z, Kwiatek MA, Pandolfino JE, Kahrilas PJ. Weak peristalsis in esophageal pressure topography: classification and association with dysphagia. Am J Gastroenterol. 2011;106:349–56
- Li Y, Xie C, Wu K-M, Chen M, Xiao Y. Motility characteristics in the transition zone in Gastroesophageal Reflux Disease (GORD) patients. BMC Gastroenterology. 2016;16:106
- Ask P, Tibbling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol1980; 238:G485–G490
- Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol 1981; 241:G129–G136
- Stoikes N, Drapekin J, Kushnir V, Shaker A, Brunt LM, Gyawali CP. The value of multiple rapid swallows during preoperative oesophageal manometry before laparoscopic anti-reflux surgery. SurgEndosc. 2012;26:3401–3407
- Bhatia SJ, Reddy DN, Ghoshal UC et al. Epidemiology and symptom profile of gastro esophgeal reflux in the Indian population: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol 2011;30: 118-127.