Prachi B Tripathi, Anjali D Amarapurkar
Departments of Pathology
T.N. Medical College & BYL Nair Charitable Hospital,
Mumbai, India
Corresponding Author:
Dr. Anjali D Amarapurkar
Email: anjali1963@gmail.com
Abstract
Introduction: Gastrointestinal tuberculosis (GITB) is a great mimicker and it is often difficult to distinguish GITB from other inflammatory lesions of the intestine.
Aim: This study was carried out with the objective of analysing the entire morphological spectrum of GITB.
Methods: A total of 110 diagnosed cases of GITB were included in the study. The diagnosis was based on the presence of acid-fast bacilli (AFB) on histology, caseating or non-caseating epithelioid cell granulomas (ECGs), evidence of tuberculosis at other extraintestinal sites, and all of these along with a complete response to anti-tuberculous treatment (ATT).
Results: The mean age was 30.9 years with M:F ratio of 1:1. On gross examination, apart from typical tuberculous lesions in the form of transverse ulcers, strictures, hyperplastic lesions and serosal tubercles, intestinal perforation (32.6%) was seen with higher frequency and ischemic bowel was also identified (7.3%). Varied morphological patterns of ECGs in the form of caseating, non-caseating, confluent, discrete and even suppurative granulomas were identified on histopathology. An important finding was the co-existence of different types of granulomas within the same case. In a significant number of cases (44.5%) granulomas were seen in a submucosal location. The predominant type of inflammation seen in the lamina propria was lymphoplasmacytic in 85.5% cases.
Conclusion: Pathologists should be aware of the entire spectrum of gross and histopathological features of GITB, so as to avoid misdiagnosis.
|
48uep6bbphidcol4|ID 48uep6bbphidvals|293 48uep6bbph|2000F98CTab_Articles|Fulltext Despite longstanding efforts to conquer tuberculosis (TB), it continues to be a major health problem in developing countries like India, where deaths due to TB account for 50/100,000 population.[1] Although the lung is the common site for primary TB infection, the gastrointestinal tract is one of the most frequent sites of extrapulmonary involvement in tuberculosis.[2] In the tropics, gastrointestinal tuberculosis (GITB) accounts for about 10% of all gastrointestinal pathologies.[3,4] Gastrointestinal TB is difficult to diagnose. Very often the clinical signs and symptoms are non- specific. Because the clinical and radiological findings of GITB mimic several diseases, such as Crohn’s disease, malignancy, amoebiasis, and Yersinia infection, it sometimes takes a long time to make an accurate diagnosis of GITB in clinical practice.[5] The histopathological diagnosis of GITB mainly rests on the identification of well-formed epithelioid cell granulomas (ECGs) with caseous necrosis. However, in the absence of caseating granulomas, it becomes difficult for the pathologist to differentiate it from other inflammatory and granulomatous lesions.[6,7] Newer diagnostic modalities such as TB PCR have now been established for the diagnosis of tuberculosis. However they have their own limitations. Needless to say, histopathology still remains the gold standard for the diagnosis of GITB in routine practice. As per age old concepts, the presence of non-caseating or necrotising granulomas rules out tuberculosis in the intestine. Also, features of GITB like intestinal perforation and gangrene of the bowel, although known are not well documented in literature. In order to evaluate the relevance of such concepts in the current scenario, this study was aimed to evaluate the entire morphological spectrum of the disease in diagnosed cases of GITB.
Methods
The study was performed at the Department of Pathology of a tertiary care hospital in Mumbai. A total of 110 diagnosed cases of gastrointestinal tuberculosis (GITB) were included in the study, over a period of 5 years (2003 to 2008). These comprised of 88 surgically resected specimens and 22 autopsies, where the cause of death was tuberculosis.
The diagnosis of GITB was based on any one of the following criteria:
1. presence of acid-fast bacilli (AFB) on histology,
2. caseating or non-caseating epithelioid cell granulomas (ECGs) on histological sections,
3. evidence of tuberculosis at other extraintestinal sites seen on radiological imaging or found at autopsy.
4. all of these along with a complete response to antituberculous treatment (ATT).
The history and clinical details of all the cases were obtained from hospital records. Evidence of extraintestinal tuberculosis was also noted either at autopsy or on radiological examination in cases of resected specimens. Gross and microscopic features of all GITB specimens were studied in detail. Representative tissue sections were evaluated for detailed histopathological findings. Staining for the demonstration of acid fast bacilli was performed on all samples using the Ziehl-Neelsen staining method.
Results
Demographic profile
As seen in Table 1, of the total 110 cases, 55 were male and 55 female with a male:female ratio of 1:1. The age range of the patients was between 2 and 78 years with a mean age of 30.9 years. The maximum number of cases, 61 (55.4%), were seen in the age group of 21 to 40 years.
Clinical Presentation
The most common clinical presentations were abdominal pain in 91 (82.7%), fever in 64 (58.2%) and weight loss in 59 (53.6%) cases. The other complaints included diarrhoea in 32 (29.1%), gastrointestinal bleeding in 6 (5.4%) and palpable abdominal lump in 2 (1.8%) cases. Of the 110 cases, 40 (36.4%) presented with subacute to acute intestinal obstruction and 36 (32.7%) presented with intestinal perforation. Evidence of tuberculosis in organs other than the intestine was found in28 (25.4%) cases. Of these, 14 (50%) cases showed evidence of pulmonary TB on imaging studies. Evidence of disseminated tuberculosis involving the gastrointestinal tract along with lungs, lymph nodes, liver, spleen, meninges or genito-urinary tract was seen at autopsy in another 14 (50%) cases.
Gross morphology
On gross examination, concurrent involvement of the ileum, caecum and ascending colon was seen most frequently in 56 (50.9%) cases. The next most common site of involvementwas the terminal ileum alone, in 43 (39.1%) cases. Other sites of involvement were the proximal ileum and jejunum in 7 (6.4%) cases, descending colon and rectum in 2 (1.8%) cases and appendix alone was involved in 2 (1.8%) cases. The most common gross morphological features identified were ulcers in 73 (66.4%) and strictures in 57 (51.8%) cases. Other gross features were perforation, serosal tubercles and ischemic bowel wall (Table 2). Hyperplastic lesions were seen in 5 (4.5%) cases, pseudopolyps in 8 (7.3%) cases and a fistula was identified in 1 (0.9%) case. The pseudopolyps were small (<0.5 cm), 1 to 3 in number and were seen adjacent to areas of ulceration. Hyperplastic lesions caused thickening of the bowel wall (1 to 2 cm) and narrowing of the lumen and were seen exclusively in the ileocaecal region.

Microscopic features
On microscopic examination, all cases showed the presence of epithelioid cell granulomas (ECGs). As seen in Table 3, caseating granulomas were seen in 68 (61.8%) cases and non-caseating granulomas in 65 (59.1%) cases. Noncaseating granulomas alone were seen in 42 (38.2%) cases. Suppurative granulomas defined by the presence of polymorphs in the centre of the granulomas were seen in 23 (20.9%) cases, of which 14 (12.7%) showed suppurative granulomas alone. Discrete granulomas were seen in 29 (26.4%) and confluent in 85 (77.3%) cases. Discrete granulomas alone were seen in 25 (22.7%) cases. Presence of lymphocyte cuffing around the granulomas was seen in 51 (46.4%) cases. Epithelioid cell granulomas were seen in the mucosal layer of the bowel wall in 61 (55.5%) cases. In the remaining 49 (44.5%) cases, the granulomas were present below muscularis mucosa. Apart from the presence of granulomas, the microscopic features that were evaluated in the histological sections were lymphoid aggregates, pyloric metaplasia, dilated submucosal lymphatics and presence of cryptitis/crypt abscesses. These were seen in 39 (35.4%), 16 (14.5%), 18 (16.4%) and 1 (0.9%) cases, respectively.
In all 110 cases, the predominant type of inflammation seen within the lamina propria was noted. The type of inflammation was evaluated at a mark away from the site of ulceration. This was predominantly lymphoplasmacytic in 94 (85.5%) cases, polymorphonuclear in 5 (4.5%) cases, eosinophilic in 2 (1.8%) cases and mixed in 9 (8.2%) cases. ZN staining for demonstration of AFB was performed on tissue sections of all 110 cases. AFB were demonstrated in 28 (25.5%) cases. Of these, 26 (92.8%) showed caseation on histology, the other two were non-caseating.
Enlarged abdominal lymph nodes were seen in 67 (65%) cases. All of these showed the presence of epithelioid cell granulomas. Of these, 60 (89.5%) showed caseation, whereas 7 (10.5%) were non-caseating. Of the 42 cases showing noncaseating granulomas in the intestine, 20 (47.6%) cases showed caseation in the lymph nodes.
HIV positive cases
HIV positivity was seen in 7 (6.4%) cases. Of these, 5 were surgical specimens which showed intestinal perforation in addition to ulcers (3 cases), strictures (2 cases) and serosal tubercles (3 cases). The remaining two were autopsy cases which showed evidence of only serosal miliary tubercles without ulceration over the mucosa. Microscopically, all the 7 cases showed presence of granulomas. Caseation was seen in 3 (42.9%) cases, while non-caseating granulomas were seen in 4 (57.1%) cases. Similarly, confluence of granulomas was seen in 3 (42.9%) cases and discrete granulomas were seen in 4 (57.1%) cases. Suppurative granulomas were seen in 1 case. Lymphocytic cuffing around the granulomas was identified in only 1 (14.2%) case. AFB could be demonstrated in 3 (42.9%) cases. Enlarged mesenteric/abdominal lymph nodes revealing the presence of caseation were seen in all 7 cases.
Discussion
Of the 110 cases of GITB, the majority, i.e. 61 (55.4%) belonged to the 21 to 40 years’ age group with male:female ratio of 1:1, which is similar to the other studies in the literature.[8,9,10] The most common clinical presentation in this study was abdominal pain, seen in 91 (82.7%) cases, followed by fever in 64 (58.2%) and weight loss in 59 (52.6%) which was also seen in the studies by Patel et al[7] and Amarapurkar et al.[11] The clinical course of 34 (30.9%) cases was chronic and insidious. Forty (36.4%) cases presented with subacute to acute intestinal obstruction and 36 (32.7%) with intestinal perforation. Similarly, a higher incidence (46.3%) of acute presentation in cases of tuberculosis was reported by Bhansali.[10] In a study by Leung et al[12] intestinal obstruction and intestinal perforation was found in 14% and 9% of the cases of intestinal TB respectively. Bolukbas et al[13] who evaluated the clinical spectrum of abdominal TB also found a 10.2% incidence of acute abdomen in cases of intestinal TB. The coexistence of intestinal TB and pulmonary TB varies from 5 to 36 %.[2,5,13] In the present study, evidence of TB in sites other than the intestine was found in 28 (25.4%) cases. The most common site of involvement in cases of GITB is the ileocaecal region.[13,14,15,16] Likewise, in this study too, the ileocaecal junction was most frequently involved (50.9%). Similar observations have also been reported by Patel et al[7] and Tandon and Prakash.[9] The appendix alone was found involved in 2 (1.8%) cases. The other causes of granulomatous appendicitis were ruled out on the basis of clinical and histopathological findings.[17] There are isolated reports in the literature describing tuberculous involvement of the appendix.[18] With regards to the site of involvement, other infective causes like Yersinia and Salmonella, which also preferentially involve the ileocaecal region, need to be ruled while considering the diagnosis of TB.[19] Also, the caecum is the most common site infected by Entamoeba histolytica.[19] In all these cases, clinical, histological and other laboratory parameters help to make the differentiation from TB. On gross examination, intestinal ulcers were seen in 73 (66.4%) cases. A majority of these were either annular or transversely placed involving the entire circumference of the bowel wall, (Figure 1) similar to characteristic tuberculous ulcers described by Tandon and Prakash.[9] A substantial number of cases, i.e. 48 (43.6%), showed skip lesions in the ileum consisting of multiple ulcers with variable length of uninvolved mucosa present in between. Skip lesions have been described as one of the gross manifestations of intestinal TB by Tandon and Prakash,[9] Amarapurkar et al[20] and Leung et al.[12]
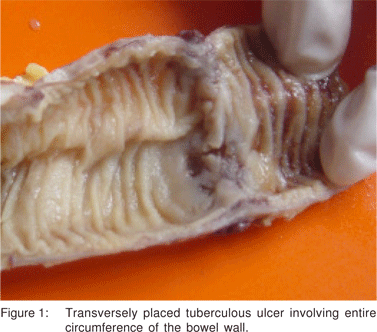
Strictures were seen in 57 (51.8%) cases and were the second most common feature seen on gross examination. Strictures have been reported in 23% cases of colonic TB by Alvares et al.6 and in 13% to 20% cases by Amarapurkar et al.[11,20]
In account on abdominal tuberculosis, Gharpure[21] in 1951 stated that ‘Perforation of a tuberculous ulcer is very rare’. Bhansali et al[22] analysed 19 cases of tuberculous intestinal perforations seen from January 1963 through July 1967. They found the incidence of perforation to be 14% of the cases of intestinal TB. Other studies have also reported intestinal perforation occurring in 1 to 15% cases of GITB.[8,10,12,23] Thus, although TB is known to cause intestinal perforation, in contrast to all previous literature, in this study intestinal perforation was seen with much higher frequency (32.7%). This is a significant finding as it puts a question mark to the age old belief that perforation of a tuberculous ulcer is rare. A majority (55.5%) of the perforations were seen in the terminal ileum which is similar to that seen in the study by Bhansali et al.[22]
Vasculitis has been described as a feature of intestinal TB in the literature.[24,25] However, ischemia identified as a gross feature in cases of GITB has not been discussed extensively. In the present study, ischemia, characterised by the dull and dusky appearance of the bowel wall was seen in 8 (7.3%) cases.
Inflammatory pseudopolyps have been described as characteristic features of ulcerative colitis[26] and also of Crohn’s disease.[27] Out of 159 cases of intestinal TB studied by Tandon and Prakash,[9] pseudopolyposis was seen in one case. Pseudopolyps were seen in 19.2% cases of GITB by Amarapurkar et al[20] and in 14% cases of colonic TB by Alvares et al.[6] In this study, pseudopolyps were seen in 8 (7.3%) cases, 7 of which occurred in the ileocaecal region.
On microscopic examination, epithelioid cell granulomas (ECGs) were seen in all cases, (Figure 2) of which 68 (61.8%) showed caseation. Caseation was seen in 40% of the cases of tuberculous enteritis in the study by Pulimood et al28 and in 64.2% cases by Patel et al.[7] However, absence of caseation does not rule out the possibility of TB.
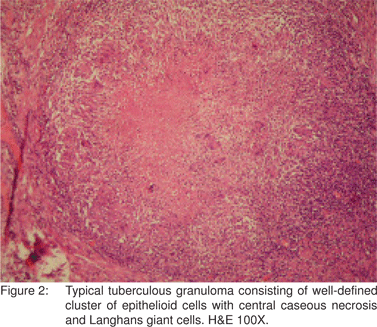
Confluence of granulomas was seen in 60% of the cases of tuberculous enterocolitis by Pulimood et al[28] and in 23.1% cases by Amarapurkar et al.[20] In the present study, confluent granulomas were seen in 85 (77.3%) cases and discrete granulomas were seen in 29 (26.4%) cases. Hence, although confluent granulomas characterise tuberculous lesions, presence of discrete granulomas does not rule out the possibility of TB.
Lymphocytic cuffing around granulomas was seen in 46.4% of cases in this study. The presence of lymphocytic cuffing is a characteristic feature seen in granulomas of tuberculosis.[9] The granulomas of Crohn’s disease have been described as characteristically naked or sarcoid-like with the absence of lymphocytic cuffing.[29]
El-Maraghi and Mair[30] have described suppurative granulomas as a histological feature of Yersinia enterocolitis. However, suppurative granulomas have also been observed in cases of ileocaecal TB by Gaffney et al.[31] Similarly, in the present study, suppurative granulomas were seen in 23 (20.9%) cases (Figure 3). Hence, as is apparent from the discussion above, various types of granulomas were observed in the histological sections. These included caseating, noncaseating, discrete, confluent and even suppurative. Also, different types of granulomas coexisted in the same case (Figure 4).
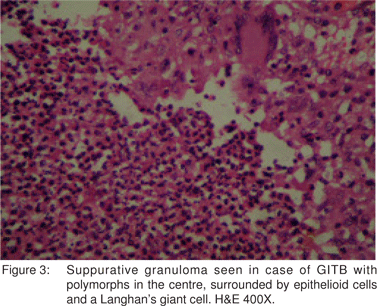
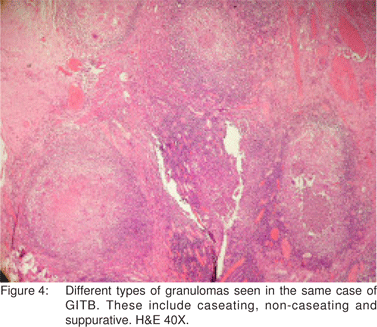
In the present study, epithelioid cell granulomas were seen in the mucosal layer of the bowel wall in 61 (55.5%) cases. In the remaining 49 (44.5%) cases, the granulomas were present deep to the mucosa. Kirsch et al[32] and Pulimood et al[28] observed granulomas in a submucosal location in 44% and 45% cases of intestinal TB, respectively. This finding is important while interpreting mucosal biopsies, where a limited amount of deeper tissue is available and where submucosally located granulomas in cases of GITB may not be sampled. Keeping this in view, the predominant type of inflammation seen in the lamina propria was evaluated in all the 110 cases of GITB and was most commonly lymphoplasmacytic, in 94 (85.5%) cases, followed by polymorphonuclear in 5 (4.5%) cases, eosinophilic in 2 (1.8%) cases and mixed in 9 (8.2%) cases. This feature needs to be further evaluated in mucosal biopsies of confirmed cases of GITB. It also needs to be evaluated in comparison with other pathologies of the GI tract.
Enlarged abdominal lymph nodes were noted in 67 (65%) cases, all of which showed epithelioid cell granulomas. Leung et al[12] found that in 27.3% cases of intestinal TB, there was caseation in the lymph nodes while it was absent in the intestinal lesions. Similarly, in the present study, 20 (18.2%) cases showed caseation in the lymph nodes along with the presence of non-caseating granulomas in the intestine.
Demonstration of acid fast bacilli in tissue sections is a definitive diagnostic method for tuberculosis. Alvares et al[6] found AFB positive in 5% cases of colonic tuberculosis. Patel et al[7] demonstrated AFB smear positivity in 40.5% cases of GITB in tissues from luminal sources. Thus even though it is an important diagnostic test for tuberculosis, the sensitivity of AFB detection in tissues is very low ranging from about 10 to 50%. AFB were demonstrated in 28 (25.5%) cases in this study. Thus, the absence of acid fast bacilli does not always rules out tuberculosis completely.
In the present study, 7 (6.4%) cases were HIV positive. Patel et al[7] found HIV seropositivity in 13% cases of confirmed GITB. Friedenberg KA et al[33] stated that the resurgence of tuberculosis, due to the AIDS pandemic may result in an increasing frequency of unusual presentations such as intestinal perforation. In the present study, of the 7 HIV positive cases, 5 were surgical specimens, all of which presented with intestinal perforation. The remaining two were autopsy cases presenting with disseminated miliary tuberculosis. The typical microscopic features of tuberculous granulomas in the form of caseation, confluence and presence of lymphocytic cuffing were seen in HIV positive cases with a lower frequency, as compared to all the cases in the study taken together. De Noronha et al[34] suggested that HIV infection alters the organisation of tuberculous granulomas by modulating the tumour necrosis factor and alters cell trafficking which leads to an impaired anti-tuberculous response.
In conclusion, the pathologist should be aware of the entire spectrum of gross and microscopic features of GITB to avoid misdiagnosis.
References
1. Anil P. Tuberculosis: a snap shot picture. Health Millions. 1995;21:8–9.
2. Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120:305–15.
3. Bhargava DK. Intestinal tuberculosis. Trop Gastroenterol. 1980;2:62–8.
4. Savita Kulkarni, Soumil Vyas, Avinash Supe, Gururaj Kadival. Use of polymerase chain reaction in the diagnosis of abdominal tuberculosis. J Gastroenterol Hepatol. 2006;21:819–23.
5. Hulnick DH, Megibow AJ, Naidich DP, Hilton S, Cho KC, Balthazar EJ. Abdominal tuberculosis: CT evaluation. Radiology. 1985;157:199–204.
6. Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005;37:351–6.
7. Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Gastrointestinal luminal tuberculosis: Establishing the diagnosis. J Gastroenterol Hepatol. 2004;19:1240–6.
8. Yilmaz A. Intestinal and peritoneal tuberculosis: changing trends over 10 years and a review of 80 patients. Can J Surg. 2005;48:131–6.
9. Tandon HD, Prakash A. Pathology of intestinal tuberculosis and its distinction from Crohn’s disease. Gut. 1972;13:260–9.
10. Bhansali SK. Abdominal tuberculosis: Experiences with 300 cases. Am J Gastroenterol. 1977;67:324–37.
11. Amarapurkar DN, Patel ND, Amarapurkar AD, Agal S, Baigal R, Gupte P. Tissue polymerase chain reaction in diagnosis of intestinal tuberculosis and Crohn’s disease. J Assoc Physicians India. 2004;52:863–7.
12. Leung VK, Law ST, Lam CW, Luk IS, Chau TN, Loke TK, et al. Intestinal tuberculosis in a regional hospital in Hong Kong: a 10-year experience. Hong Kong Med J. 2006;12:264–71.
13. Bolukbas C, Bolukbas FF, Kendir T, Dalay RA, Akbayir N, Sokmen MH, et al. Clinical presentation of abdominal tuberculosis in HIV seronegative adults. BMC Gastroenterol. 2005;5:21.
14. Horvath KD, Whelan RL. Intestinal tuberculosis: Return of an old disease. Am J Gastroenterol. 1998;93:692–6.
15. Wadhwa N, Agarwal S, Mishra K. Reappraisal of abdominal tuberculosis. J Indian Med Assoc. 2004;102:31–2.
16. Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–99.
17. Timmcke AE. Granulomatous appendicitis: Is it Crohn’s disease? Report of a case and review of literature. Am J Gastroenterol. 1986;81:283–7.
18. Morrison H, Mixter CG, Schelesinger MJ, Ober WB. Tuberculosis localized in the vermiform appendix. N Engl J Med. 1952;246:329–31.
19. Lamps LW. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50:55–63.
20. Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–6.
21. Gharpure KC. Abdominal tuberculosis. J Indian Med Assoc. 1951;21:57–61.
22. Bhansali SK, Desai AN, Dhaboowala CB. Tuberculous perforation of the small intestine: A clinical analysis of 19 cases. J Assoc Physicians India. 1968;16:351–5.
23. Schulze K, Warner HA, Murray D. Intestinal tuberculosis: experience at a Canadian teaching institution. Am J Med. 1977;63:735–45.
24. Shah P, Ramakantan R. Role of vasculitis in the natural history of abdominal tuberculosis- evaluation by mesenteric angiography. Indian J Gastroenterol. 1991;10:127–30.
25. Mapstone NP, Dixon MF. Vasculitis in ileocaecal tuberculosis: similarities to Crohn’s disease. Histopathology. 1992;21:477–9.
26. Kirks DR, Currarino G, Berk RN. Localized giant pseudopolyposis of the colon. Am J Gastroenterol. 1978;69:609–14.
27. Kahn E, Daum F. Pseudopolyps of the small intestine in Crohn’s disease. Hum Pathol. 1984;15:84–6.
28. Pulimood AB, Ramakrishna BS, Kurian G, Peter S, Patra S, Mathan VI,et al. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn’s disease from tuberculosis. Gut. 1999;45:537–41.
29. Price AB, Morson BC. Inflammatory bowel disease. The surgical pathology of Crohn’s disease and ulcerative colitis. Hum Pathol. 1975;6:7–29.
30. El-Maraghi NR, Mair N. The histopathology of enteric infection with Yersinia pseudotuberculosis. Am J Clin Pathol. 1979;71:631–9.
31. Gaffney EF, Condell D, Majmudar B, Nolan N, McDonald GS, Griffin M, et al. Modification of caecal lymphoid tissue and relationship to granuloma formation in sporadic ileocaecal tuberculosis. Histopathology. 1987;11:691–704.
32. Kirsch R, Pentecost M, Hall Pde M, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn’s disease and intestinal tuberculosis. J Clin Pathol. 2006;59:840–4.
33. Friedenberg KA, Draguesku JO, Kiyabu M, Valenzuela JE. Intestinal perforation due to Mycobacterium tuberculosis in HIV-infected individuals; report of two cases. Am J Gastroenterol. 1993;88:604–7.
34. De Noronha AL, Bafica A, Nogueira L, Barral A, Barral-Netto M. Lung granulomas from Mycobacterium tuberculosis/HIV-1 coinfected patients display decreased in situ TNF production. Pathol Res Pract. 2008;204:155–61.
|