Gastroesophageal reflux disease (GERD) is a chronic relapsing condition, which significantly affects the healthrelated quality of life.[1,2] Reflux of gastric contents into the esophagus causes injury to its mucosa resulting in erosive esophagitis. This is associated with symptoms such as heartburn, regurgitation and chest pain. Complications such as esophageal stricture, Barrett’s metaplasia and esophageal adenocarcinoma may develop if GERD remains untreated for a long time.
GERD is often treated with acid suppressive or neutralizing drugs such as antacids, histamine receptor antagonists (H2RA) and proton pump inhibitors (PPI) for prolonged periods. Among these, PPI are the most popular drugs in current practice. Though long-term use of PPI is considered safe in the management of patients with GERD, prolonged PPI treatment is known to cause gastric atrophy, parietal cell hyperplasia and carcinoid tumours in animals.[3,4] Such complications in humans have not been reported. We report the first case of a patient who developed gastric atrophy and intestinal metaplasia (IM) while on long-term PPI use for probable GERD.
Case Report
A 74-year old man presented with a history of heartburn and regurgitation for which he had been taking high dose PPI (pantoprazole 40-80 mg/d) for the last 20 years with good symptom control. Upper gastrointestinal (UGI) endoscopy, 24- h dual channel pH-metry and esophageal manometry were planned for. The patient was asked to stop taking all drugs including PPI for a duration of one month before these investigations were performed.
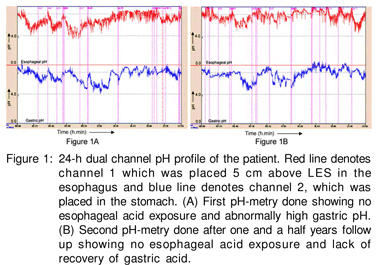
Esophageal manometry performed using an 8-channel water perfusion manometry system (RedTech Biomedicals, CA, USA) showed normal body motility and lower esophageal sphincter pressure. 24-h dual channel pH-metry performed using Naik-II pH-metry system (RedTech Biomedicals, CA, USA) showed percentage reflux time (calculated as percentage time esophageal pH recorded below 4) of 0.30% (approx. 4.32 minutes; normal <5%) of total 24-hour study. A total of 11 reflux episodes were recorded, of these none were longer than 5 minutes each. The average esophageal pH was 6.15. However, average gastric pH revealed achlorhydria (pH 6.57, Figure 1A). This was an unusual finding even after stopping PPI for one month.
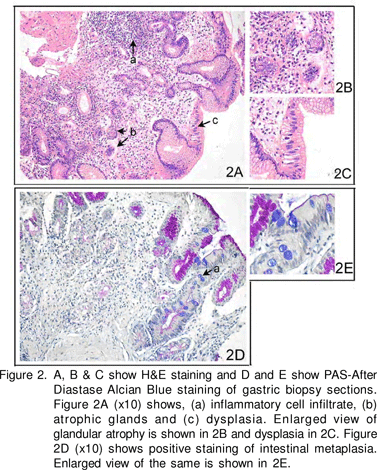
UGI endoscopy showed prominent submucosal vessels in the fundus and body. Antrum appeared normal. No hiatus hernia was found. Rapid urease test (RUT) on biopsies from antrum and body was negative. Histopathological examination of antral and corpus biopsies stained with H&E (hematoxylin and eosin), Periodic Acid Schiff’s and Alcian Blue (PAS-AD AB) (Figure 2) showed diffuse pyloric gland metaplasia with widely spaced mucous glands. The intervening lamina propria showed an excess of mixed inflammatory cell infiltrate and marked condensation of reticulin fibres around the glands. Focal intestinal metaplasia (IM) and an occasional lymphoid follicle were also seen. Antral biopsy also showed focal atrophy and mixed inflammatory cell infiltrate in lamina propria. No spiral organisms were seen. Anti-H.pylori IgG ELISA however, was positive [Genesis Diagnostics, Cambridgeshire, UK; positive =6.25 IU/mL (cut-off value to classify positive and negative as per manufacturer’s instruction)].
All anti-secretory drugs were discontinued and prokinetics started. On follow up, the patient felt numbness and tingling in the feet and postural imbalance while walking. The serum level of vitamin B12 was low and parenteral injections of B12 were given, which resulted in disappearance of these symptoms.
A second dual 24-h dual channel pH metry after one year of follow up still showed high gastric pH (average 6.76, Figure 1B). Thus, his gastric acid secretion did not revert back despite withdrawal of PPI suggesting irreversible gastric atrophy. The patient remained well without PPI and prokinetics and vitamin B12 injections were continued during a one and halfyear follow up.
Discussion
PPI has been used as a potent acid suppressive drug in controlling acid-peptic diseases (such as GERD) effectively. PPIs inhibit hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase) activity on the luminal surface of the parietal cell, effectively blocking the final common pathway for gastric acid secretion, thus causing hypochlorhydria. Reports on complications like gastric neoplasm following prolonged and high dose of PPI use has been shown in animal models only. It has been reported in the literature that prolonged PPI administration in rats is associated with the development of carcinoid tumours of enterochromaffin-like (ECL) cells in the gastric corpus.[3,5] This is due to gastrin, which becomes chronically elevated in animals subjected to prolonged and profound hypochlorhydria.[6] In humans, hypergastrinemic states such as Zollinger-Ellison syndrome and atrophic gastritis are associated with an increased risk of ECL-cell carcinoid tumours.[4,7] Also, hypergastrinemia is the dominant agent acting as a promoter in all steps (hyperplasia-dysplasianeoplasia) of the tumourigenic sequence.[8]
Such observations have raised concern that prolonged use of potent acid suppressive drugs in human might predispose to gastric malignancy. However, till date, there is no report on such complications in humans. Though, a study on the effect of long term (up to 10 years) PPI blockade on human gastric mucosa9 showed a general amelioration of antral gastritis without significant changes of atrophy or IM, contrasted with the worsening of gastritis and gland atrophy seen in the oxyntic mucosa of patients with GERD (but not gastric or duodenal ulcer) in the presence of H. pylori infection. Besides, they confirm a substantial safety of long-term PPI therapy in relation to neoplastic changes in the exocrine and endocrine human stomach.[9] Therefore, ours is the first report showing the development of gastric atrophy and IM in a patient on long-term (20 years) and high-dose PPI.
These findings suggest a possible role of PPI in causing the precancerous gastric lesion i.e. IM. Another study on
patients with Barrett’s esophagus treated with PPI and H2RA for 2-y showed significant increase in the parietal cell count, ECL cell count and fasting serum gastrin levels in the group treated with PPI, though in the H2RA treated group, there was no increase in the ECL cell count or serum gastrin level, though the parietal cell count decreased. In both groups, there was no change in the thickness of the mucosa.[10] Thus, PPI has more adverse effect than H2RA on the gastric mucosa.
Deficiency of vitamin B12 also suggests severe gastric atrophy in our patient. This was due to the absence of intrinsic factor, a glycoprotein secreted by the parietal cells of the stomach, which is essential for the absorption of vitamin B12. This deficiency may remain subclinical or in severe state may result in anaemia, pancytopenia, peripheral neuropathy and posterior spinal column neuropathy.[11,12]
A second 24-h dual channel pH-metry after one and a half years of follow up in this patient still revealed a high gastric pH. This may be due to the complete disappearance of parietal cells. This further indicates persistent severe gastric atrophy It is also known that H.pylori infection may interfere with the acid suppressive therapies used for treating GERD.[13] Longterm therapy of GERD in H.pylori infected patients may lead to rapid progression of atrophic gastritis, IM and dysplasia, and increase the risk of developing gastric cancer. Tissue-based tests for the detection of H.pylori infection such as RUT and histology tested negative in our patient possibly due to gastric atrophy and IM;[14] in such a situation serology is the only definitive test to show the presence of H.pylori infection.[15] We believe that in our patient, gastric atrophy associated with H.pylori infection may have been exaggerated by the prolonged use of PPI. This is supported by the studies which showed proximal migration of H. pylori and gastritis in patients with H.pylori infection treated with PPI.[16] Thus, corpus gastritis may further lead to the destruction of parietal cells, causing hypo or achlorhydria.[17] However, the role of host genetic factors and environmental factors in the progression towards precancerous gastric lesions or even gastric cancer in humans cannot be discounted.[18]
Therefore, high dose and prolonged use of PPI in humans may be viewed cautiously as this may be a possible risk factor leading to potentially precancerous gastric lesions such as gastric atrophy and IM.
Acknowledgement
This work was supported by an intramural grant to UCG from Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
One of the authors, Dipti Chourasia, thanks the Indian Council of Medical Research (ICMR) for providing her the fellowship.
References
1. Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med. 1998;104:252–8.
2. Holtmann G. Reflux disease: the disorder of the third millennium. Eur J Gastroenterol Hepatol. 2001;13:S5–11.
3. Karnes WE Jr, Walsh JH. The gastrin hypothesis. Implications for antisecretory drug selection. J Clin Gast roenterol. 1990;12:S7–12.
4. Gilligan CJ, Lawton GP, Tang LH, West AB, Modlin IM. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol. 1995;90:338–52.
5. Hakanson R, Bottcher G, Sundler F, Vallgren S. Activation and hyperplasia of gastrin and enterochromaf f in-like cells in the stomach. Digestion. 1986;35:Suppl 1:23–41.
6. Eissele R, Arnold R. [Risk for developing tumors in therapy with the proton pump inhibitor omeprazole]. Versicherungsmedizin. 1993;45:126–9.
7. Bordi C, D’Adda T, Azzoni C, Pilato FP, Caruana P. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol. 1995;19 Suppl 1:S8–19.
8. Bordi C, D’Adda T, Azzoni C, Ferraro G. Pathogenesis of ECL cell tumors in humans. Yale J Biol Med. 1998;71:273–84.
9. Lamberts R, Brunner G, Solcia E. Effects of very long (up to 10 years) proton pump blockade on human gastric mucosa. Digestion. 2001;64:205–13.
10. Nishi T, Makuuchi H, Weinstein WM. Changes in gastric ECL cells and parietal cells after long-term administration of high-dose omeprazole to patients with Barrett’s esophagus. Tokai J Exp Clin Med. 2005;30:117–21.
11. Carmel R. Cobalamin, the stomach, and aging. Am J Clin Nutr. 1997;66:750–9.
12. Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin def iciency. Medicine (Baltimore).1991;70:229–45.
13. Vigneri S, Termini R, Savarino V, Pace F. Review article: is Helicobacter pylori status relevant in the management of GORD? Aliment Pharmacol Ther. 2000;14:31–42.
14. Karnes WE Jr, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW, et al. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167–74.
15. Kang HY, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51:2310–5.
16. ogan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36:12–6.
17. McColl KE. Helicobacter pylori and acid secretion: where are we now? Eur J Gastroenterol Hepatol. 1997;9:333–5.
18. Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346–51.