

Discussion
Our study demonstrated a higher prevalence of DM in CHC, even in non- cirrhotic patients; lower incidence of traditional risk factors in patients with DM and CHC; no relation between prevalence of DM and HCV genotype; and temporal relationship of HCV to DM. These all support a role of HCV in the aetiopathogenesis of DM.
Our study showed increased prevalence of DM in CHC compared to CHB, which is in accordance with previous reports.[4,5]
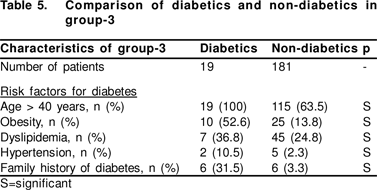
Our study could not identify patients with increased risk factors for the development of DM in CHC. In previous series, HCV was shown to increase the chances of development of DM in the presence of traditional risk factors: non-white race,[3] genotype 2a or 1a,[4] severity of underlying liver disease or presence of cirrhosis,[1,2,5] increased age,[3,4,5,6] male sex,[6] obesity,[3,6] low socioeconomic class,3 previous interferon treatment,[21] or family history of DM.2 In a recent series, it was shown that diabetics in HCV infection did not show classical phenotypic correlates, but presented at a younger age, and had lower rates of obesity and dyslipidemia.[22] Together with our series, these findings support the concept that DM in CHC represents two types of patients i.e. one related to traditional risk factors and one related to the HCV infection itself.
In our series, there was no effect of HCV genotypes on the development of DM. Most extrahepatic manifestations are not genotype-specific.[1]
Temporal relationship with HCV infection and development of DM is difficult to estimate accurately, as both disorders have insidious onset. Our study showed that in patients where the exact duration of both DM and HCV infection was known, HCV infection preceded the development of DM by a decade, even in the absence of traditional risk factors.
Diabetics in our series had increased prevalence of aminotransferase activity, cirrhosis and steatosis, thereby suggesting a role of DM in the progression of liver disease of any cause. Non-alcoholic steatosis is considered a part of the metabolic syndrome and is closely associated with IR and DM. [23] In our study, diabetics with CHC were more likely to have steatosis than were CHB patients, this was in accordance to previous reports.[24]
In our study, steatosis was seen more often in CHC, even when there was increased prevalence of factors responsible for IR in CHB. This was in accordance with previous series as well.[25]
HCV causes IR either by inducing tumour necrosis factor- _,[26] or by altering insulin-signalling pathways.[27] Also HCV induces intrahepatic fat accumulation (steatosis) consequent either of direct effect on hepatocyte,[28] or of hypobetalipoprotienemia induced by HCV[29] or of indirect effect mediated through IR.[10] Steatosis in turn increases IR further[,30] resulting in a vicious cycle, which leads ultimately to the development of DM.[31] Although high ferritin levels are thought to be the result of HCV and are linked to IR, fibrosis and worsening of diabetes,[26] recent studies have shown high ferritin levels to be due to diabetes itself rather than HCV.[32] In addition, a direct cytopathic effect of HCV on pancreatic islet cells has been recently demonstrated[.33] The role of HCVinduced autoimmunity is unlikely to play a major role in the pathogenesis of DM.[4]
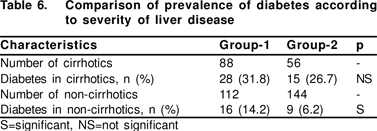
All the cirrhotics in our study had increased prevalence of DM regardless of aetiology (whether CHC or CHB). Ourfindings were in accordance with few series,[14] but were contrary to other previous series where cirrhotics with HCV infection were more likely to be diabetics compared to other aetiologies.5 All cirrhotics are predisposed to IR and thereby DM.
As non-cirrhotics also have increased prevalence of DM in CHC, it is unlikely that DM in HCV infection reflects only the severity of underlying liver disease, as was suggested by a few authors.[4] Our results were in accordance with previous series showing increased DM in non-cirrhotic HCV infection.[3,27,32]
In conclusion, HCV appears to be a predisposing and preceding factor for the development of DM, even in noncirrhotics and presents typically, i.e. without the presence of traditional risk factors.
References
1. Agnello V, De Rosa FG. Extrahepatic disease manifestations of HCV infection- some current issues. J Hepatol. 2004;40:341–52.
2. Zein CO, Levy C, Basu A, Zein NN. Chronic hepatitis C and type II diabetes mellitus- a prospective cross-sectional study. Am J Gastroenterol. 2005;100:48–55.
3. Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–9.
4. Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–33.
5. Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, et al. Further evidence for an association between non- insulin dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–63.
6. Grimbert S, Valensi P, Levy-Marchal C, Perret G, Richardet JP, Raffoux C, et al. High prevalence of diabetes mellitus in patients with chronic hepatitis C- a case-control study. Gastroenterol Clin Biol. 1996;20:544–8.
7. Mora PF. Post-transplantation diabetes mellitus. Am J Med Sci. 2005;329:86–94.
8. Abbott KC, Lentine KL, Bucci JR, Agodoa LY, Koff JM, Holtzmuller KC, et al. Impact of diabetes and hepatitis af ter kidney transplantation on patients who are affected by hepatitis C virus. J Am Soc Nephrol. 2004;15:3166–74.
9. Tanaka H, Shiota G, Kawasaki H. Changes in glucose tolerance after interferon-alpha therapy in patients with chronic hepatitis C. J Med. 1997;28:335–46.
10. Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and f ibrosis progression. Gastroenterology. 2003;125:1695–704.
11. Iyer SR. Type 2 diabetes mellitus express highway, where is the ‘U’ turn? J Ass Physc India. 2003;51:495–500.
12. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8.
13. de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes- the Verona Diabetes Study. Diabetes Care. 1999;22:756–61.
14. Mangia A, Schiavone G, Lezzi G, Marmo R, Bruno F, Villani MR, et al. HCV and diabetes mellitus- evidence for a negative association. Am J Gastroenterol. 1998;93:2363–7.
15. Sotiropoulos A, Peppas TA, Skliros E, Apostolou O, Kotsini V, Pappas SI. Low prevalence of hepatitis C virus infection in Greek diabetic Patients. Diabetes Med. 1999;16:250–2.
16. Arankalle VA. Epidemiology of HCV infection in India: a comprehensive analysis. In Hepatitis B and C- carrier to cancer. Sarin SK, Okuda K eds. Haurcourt India Pvt Ltd. 2002;201–18.
17. Amarapurkar DN. Is diabetes mellitus an extrahepatic manifestationof hepatitis C infection? Indian J Gastroenterol. 1999;18:A43.
18. Duseja A, Sharma S, Nitin, Chaudhry S, Dhiman RK, Chawla Y, et al. Prevalence of diabetes mellitus in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2004;19:A711–2.
19. Amarapurkar D, Dhorda M, Kirpalani A, Amarapurkar A, Kankonkar S. Prevalence of hepatitis C genotypes in Indian Patients and their clinical significance. J Ass Physc India. 2001;49:983–5.
20. Amarapurkar D, Das HS. Chronic liver disease in diabetes mellitus. Tropical Gastroenterol. 2002;23:3–5.
21. Imano E, Kanda T, Ishigami Y, Kubota M, Ikeda M, Matsuhisa M, et al. Interferon induces insulin resistance in patients with chronic active hepatitis C. J Hepatol. 1998;28:189–93.
22. Behrendt CE, Ruiz RB. Hyperglycemia among persons with hepatitis C- not the classical diabetic phenotype. Diabetes Research Clinc Practice. 2006;71:68–74.
23. Amarapurkar DN, Patel ND. Clinical spectrum and natural history of non-alcoholic steatohepatitis with normal alanine aminotransferase values. Tropical Gastroenterology. 2004;25:130-4.
24. Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Host and disease specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198–206.
25. Sanyal AJ, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Stravitz ML, et al. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064–71.
26. Elsammak M, Refai W, Elsawaf A, Abdel-Fattah I, Abd Elatti E, Ghazal A. Elevated serum tumour necrosis factor alpha and ferritin may contribute to the insulin resistance found in HCV positive Egyptian patients. Cur rent Med Research Opinion. 2005;21:527–34.
27. Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, et al. hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signalling 3. Am J Pathol 2004;165:1499–508.
28. Lerat H, Honda M, Beard MR, Loesch K, Sun J, Yang Y, et al. Steatosis and liver cancer in transgenic mice expressing the st ructural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–65.
29. Serfaty L, Andreani T, Giral P, Carbonell N, Chazouilleres O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia- a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–34.
30. Chou CJ, Haluzik M, Gregory C, Dietz KR, Vinson C, Gavrilova O, et al. WY14,643, a peroxisome proliferatoractivated receptor alpha (PPARalpha) agonist, improves hepatic and muscle steatosis and reverses insulin resistance in lipoatrophic A-ZIP/F- 1 mice. J Biological Chem. 2002;277:24484–9.
31. Noto H, Raskin P. Hepatitis C infection and diabetes. J Diabetes Complications. 2006;20:113–20.
32. Lecube A, Hernandez C, Genesca J, Esteban JI, Jardi R, Garcia L, et al. Diabetes is the main factor accounting for the high ferritin levels detected in chronic hepatitis C virus infection. Diabetes Care. 2004;27:2669–75.
33. Masini M, Campani D, Boggi U, Menicagli M, Funel N, Pollera M, et al. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28:940–1.