René Agustín Flores-Franco, Jorge Iván Carrillo-Miranda, Claudia Castañeda-Martínez, Antonio Salas-Muñoz
Universidad y García Conde Av., s/n
Col. Centro, CP 31000
Chihuahua, Chih., México
Corresponding Author:
René Agustín Flores-Franco
E-mail: rflores99@prontomail.com
48uep6bbphidvals|217 48uep6bbphidcol2|ID 48uep6bbph|2000F98CTab_Articles|Fulltext Amoebic liver abscess (ALA) is frequently observed in tropical countries and represents the most common extraintestinal manifestation of amoebiasis. As a potentially fatal disease, it requires prompt diagnosis and treatment since early intervention generally improves survival and abates morbidity. After the suitable treatment of ALA the liver has a great ability for regenerate and exceptionally may persist as residual cavity from few months to indefinitely.[1,2,3] Generally the healing stage of ALA can be appreciated on ultrasound and echogenic shadows and calcifications are seen; these are sometimes confounded by other forms of localised liver pathology.[4] Factors influencing the healing time from ALA such as intrathoracic complications may also affect the development of complications. Cameron5 analysed serial chest x-rays from patients with hepatopulmonary amoebiasis and described abnormalities in the diaphragm and pleural contour which persisted indefinitely following the completion of therapy.
Here, we describe an unusual case where in a child with hepatic mass, abutting the right diaphragm, images of false herniation of the liver were seen which manifested as a late sequel from previous ALA complicated with ipsilateral empyema thoracis.
Case report
An 8 year-old Mexican mestizo girl was admitted to the hospital for an episode of respiratory tract infection. During routine medical examination, a mass shadow in the right lower lung field adjacent to the diaphragm was incidentally seen on chest x-ray; and continuity between this mass and the liver, with similar density numbers, was demonstrated on consecutive computed tomography (CT).
Subsequently, a transthoracic guided core-needle biopsy of the intrathoracic mass reported normal liver tissue findings. Serology for hepatitis viruses B and C was negative and the serum alpha-fetoprotein (AFP) level was 1.37 ng/mL. Although Tc-99m scintigram and magnetic resonance (MR) corroborated a liver source do not rule out herniation because was observed an apparently positive hump sign (Figure 1).
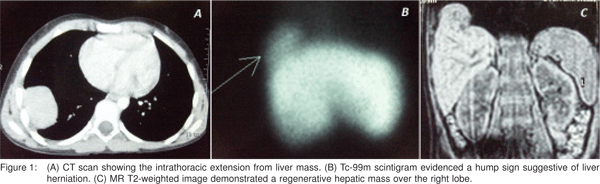
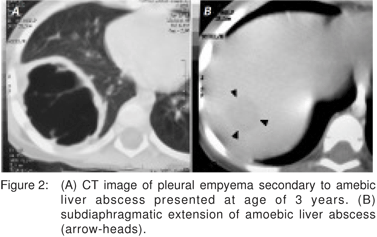
Her medical records stated that she had undergone open right-sided decortication of loculated pleural empyema associated with a 4.4 cm x 2.5 cm x 2.2 cm right lobe liver abscess, five years ago (Figure 2). At that time, the amebic serology test was positive (1:4090) and after a full course of metronidazole therapy the patient recovered without significant complication.
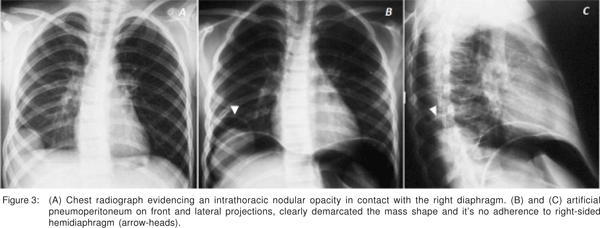
Because the patient had history of two potential events that could disrupt the diaphragm surface such as subdiaphragmatic extension of an inflammatory liver process and a thoracic surgery performed earlier, we initially considered the diagnosis of liver herniation through a perforated diaphragm. The thoracic surgeon performed under local anesthesia a diagnostic artificial pneumoperitoneum followed by plain abdominal radiographs in an upright position. This diagnostic test clearly demonstrated the extra-hepatic extension of the regenerated mass and its relation to the righthalf of the diaphragm (Figure 3).
After six-months of follow-up investigations showed no increase in serum AFP levels and there was not further variation in the size and shape of the lesion on chest radiographs.
Discussion
We consider this as the first reported case of ALA which during the late healing process resulted in a mass with extrahepatic extension. The diaphragm’s physical influence on the regenerative process of the liver may be inferred from recent work in animal models6 and from some reported cases of ectopic liver proliferation through diaphragmatic tears.[7,8,9,10] Theoretically, the localised atrophy that accompanies partial diaphragmatic eventeration is a result of pressure differential between the abdominal and thoracic cavities. As the weakened area of the diaphragm protrudes, the underlying hyperplasic liver tissue can fill the bulge resulting in its resembling an intrathoracic tumour because acute angles with the chest wall are seen on imaging studies11. In this setting the diaphragmatic eventration can also mimic a rupture. The hump sign is a variant of the collar sign and occurs as a result of a portion of liver herniating through the diaphragm, forming a humpshaped mass as observed on CT and MR images of our patient. Nevertheless, Rees et al[12] obtained a sensitivity of only 50% with this sign when CT sagittal and coronal reconstructions were used.
The role of pneumoperitoneum as an imaging tool has been substituted by less invasive technology. We only considered its use because of availability of a thoracic surgeon with expertise in this area. The use of pneumoperitoneum as an imaging tool contributed to the diagnosis of eventrated liver mass, permitted an evaluation of the presence and degree of extension of the lesion into surrounding structures, as well as the existence of adhesions between the liver and diaphragm.[13,14]
Benign tumors that can cause diagnostic dilemmas for the pathologist in the analysis of hepatic fine-needle aspiration and core biopsies include liver cell adenoma, focal nodular hyperplasia, and other regenerative nodules.[15] We assumed that our patient had a regenerative condition but differential diagnosis should also include ectopic liver proliferation and accessory lobe of the liver. Nevertheless, the absence of pedicle and a clear relation with the documented antecedent of ALA aided to discard such diagnostic possibilities.
Similar to the treatment of focal nodular hyperplasia of the liver in children[16], we finally recommended a conservative management for our patient and surgery to be performed if a symptomatic compression of adjacent organs or an increase size of the lesion occured.
In conclusion, the healing of a complicated ALA with thoracic spread has not been well studied and the present case report would be an example of transdiaphragmatic influences over an underlying post-necrotic liver regeneration process.
References
1. Ralls PW, Quinn MF, Boswell WD, Colletti PM, Radin DR, Halls J. Patterns of resolution in successfully treated hepatic amebiasis abscess: Sonographic evaluation. Radiology. 1983;149:541–3.
2. Ahmed L, Salama ZA, el Rooby A, Strickland GT. Ultrasonographic resolution time for amebic liver abscess. American Journal of Tropical Medicine and Hygiene. 1989;41:501–4.
3. Sharma MP, Dasarathy S, Sushma S, Verma N. Long term followup of amebic liver abscess: clinical and ultrasound patterns of resolution. Tropical Gastroenterology. 1995;16:24–8.
4. Blessmann J, Le Van A, Tannich E. Hepatic ultrasound in a population with high incidence of invasive amebiasis: evidence of subclinical, self-limited amoebic liver abscess. Trop Med Int Health. 2003;8:231–3.
5. Cameron EW. The treatment of pleuropulmonary amebiasis with metronidazole. Chest. 1978;73:647–50.
6. Langwieler T, Fiegel H, Alaamian M, Mann O, Beshir I, Izbicki J, et al. The relationship of diaphragmatic defect, liver growth, and lung hypoplasia in nitrofen-induced congenital diaphragmatic hernia in the rat. Pediatr Surg Int. 2004;20:509–14.
7. Lasser A, Wilson GL. Ectopic liver tissue mass in the thoracic cavity. Cancer. 1975;36:1823–6.
8. Bedii Salman A. Left-sided congenital diaphragmatic hernia associated with intrathoracic ectopic liver lobule. Eur J Cardiothorac Surg. 2002;21:558–60.
9. Yoshino I, Yamaguchi M, Kameyama T, Takuro K, Osoegawa A, Yohena T, et al. Extension of liver tissue into the thorax following a right extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Thorac Cardiovasc Surg. 2006;12:355–7.
10. Huang CS, Hsu WH, Hsia CY. Supradiaphragmatic ectopic liver: Delayed traumatic hepatic hernia mimics pulmonary tumor. Thorac Cardiovasc Surg. 2007;55:271–2.
11. Yeh HC, Halton KP, Gray CE. Anatomic variations and abnormalities in the diaphragm seen with US. Radiographics. 1990;10:1019–30.
12. Rees O, Mirvis SE, Shanmuganathan K. Multidetector-row CT of right hemidiaphragmatic rupture caused by blunt trauma: a review of 12 cases. Clin Radiol. 2005;60:1280–89.
13. Trimble HG, Leftwich WB. The diagnostic use of artificial pneumoperitoneum. Dis Chest. 1955;28:282–9.
14. Ellman B, McLeod IN, Powell SJ. Diagnostic pneumoperitoneum in amoebic liver abscess. Br Med J. 1965;2:1406–7.
15. [No Authors Listed]. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–93.
16. Okada T, Sasaki F, Kamiyama T, Nakagawa T, Nakanishi K, Onodera Y, et al. Management and algorithm for focal nodular hyperplasia of the liver in children. Eur J Pediatr Surg. 2006;16:235–40.
|