48uep6bbphidcol2|ID
48uep6bbphidvals|1941
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Portal annular pancreas (PAP), also known as circumportal pancreas is a rare fusion anomaly of the pancreas with a varying incidence reported in literature ranging from 0.8 to 2.5%.1-4 It is characterized by a rind of pancreatic parenchyma, which encircles the portal vein (PV) or the superior mesenteric vein (SMV) and fuses with the body of the pancreas.
One of the earliest reports of this fusion anomaly was reported by Suguira et al. in 1987, where they came across a case where the pancreatic parenchyma was found to be enveloping the superior mesenteric vessels during surgery.5 Though asymptomatic and incidentally detected in the majority of patients, it can be mistaken for a locally advanced pancreatic head mass. It also has crucial implications in patients planned for pancreatic operations.2,6 We have encountered both these scenarios in our clinical practice. Since there is a paucity of literature discussing this entity, probably due to its rarity, we undertook a study to determine the prevalence of PAP in a cohort of patients attending a tertiary care referral centre in South India and assess how frequently this was identified and reported in a routine CT scan report.
Materials and Methods
A retrospective observational study was performed on consecutive contrast-enhanced multi-detector computed tomography (MDCT) scans of the abdomen done in our institution. The abdominal CT scans were performed from Jan 1, 2018 to Feb 7, 2018 for various indications. Based on an expected prevalence of 2.4% according to a previous systematic review,2 precision of 1% and z-value for 95% confidence interval of 1.96, we calculated the sample size to be 940 subjects.
The demographic profile of the patients and indication for abdominal CT examination were recorded. The images were evaluated for the presence of PAP. PAP was classified into suprasplenic, infrasplenic, or mixed variants depending on the site of the parenchymal fusion – superior, inferior, or on either side of the splenoportal confluence respectively. Patients who had associated pancreatic pathology like acute/chronic pancreatitis / pancreatic focal lesion and those who subsequently had to undergo surgery were noted.
We also reviewed the radiology reports of these patients on our Picture Archival and Communication System (PACS) with regard to mention of this variant in the report.
Contrast-enhanced MDCT scans of the abdomen and pelvis were performed using the following machines – 1) 64 slice GE discovery 750 HD, Milwaukee, WI, USA scanner with a tube voltage of 100 Kv, automatically controlled tube current, pitch 0.98 and reconstructed slice thickness of 2.5-5 mm. 2) 16 slice Siemens Somatom Emotion, Essen, Germany scanner with tube voltage 110-130Kv, automatically controlled tube current, pitch 1.2, and reconstructed slice thickness of 3-5mm. The patients were given intravenous (IV) iodinated contrast media with an iodine concentration of 300 mg per millilitre at a dose of 1.5 ml/kg using a pressure injector at an injection rate of 3 ml/sec. The arterial phase imaging was done at about 20-25 seconds after injection of IV contrast followed by a venous phase imaging done at 55-60 seconds after IV contrast injection.
A descriptive statistical analysis was done. Categorical variables were presented as frequencies/percentages and quantitative variables as median with range.
Our institutional review board approved the study.
Results
A total of 1004 cases were studied. We excluded four patients in whom we could not assess the course of the portal vein. Three of these patients had portal vein thrombosis, and one had neuroblastoma causing significant displacement of the pancreas and portal vein. Out of the 1000 patients included in the final analysis, 564 were males, and 436 were females. The median age of the study group was 47 years (range 1-93 years). PAP was present in 25 patients (2.5%). The male: female ratio was 8:17. Suprasplenic variant of PAP was found in 20/25 (80%), and the rest were infrasplenic in 5/25 (20%) patients. There was no mixed variant morphology. One out of these 25 patients had acute pancreatitis, while the remaining patients had no pancreas related pathology. The indications for abdominal CT scans in these patients are summarised in Table 1. A review of the radiology reports of these 25 patients revealed that PAP was mentioned in only one of the reports (4%). None of these patients were planned for a pancreatic operation in the near future.
Discussion
A variety of developmental anomalies of the pancreas are encountered in clinical practice. Conditions like pancreas divisum, annular pancreas, and PAP constitute important fusion anomalies of the pancreas.7 The pancreas develops from the ventral and dorsal bud of the primitive foregut. The ventral bud typically rotates posteriorly fusing with the dorsal bud during the 7th week of gestation. In PAP, this fusion occurs to the left of the superior mesenteric vein and the portal vein and hence the uncinate process encircles the portal vein or the superior mesenteric vein and fuses with the dorsal surface of the pancreatic body.7
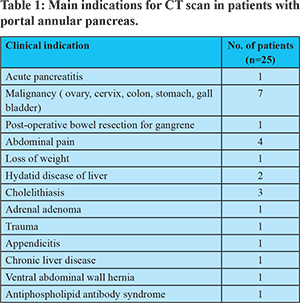
PAP can be diagnosed on CT by demonstrating a continuity of pancreatic parenchyma extending from the uncinate process to the posterior surface of the body of the pancreas, thus encircling the portal vein or SMV.
The prevalence of PAP obtained in our study (2.5%) was similar to that observed by Ishigami et al. in their study on CT scans of liver transplant donors.1 A systematic review performed by Harnoss et al., in their mixed cohort, which included case reports, reported an overall prevalence of 2.4%.2 Other institutional studies on review of CT scans have reported much lower frequencies of 1.14% by Karasaki et al.3 and 0.8% by Yilmaz and Celik.4 To the best of our knowledge, there has been no study in published literature assessing the prevalence of portal annular pancreas from the Indian subcontinent. Female preponderance was noted in our study similar to Karasaki et al.3 The systematic review performed by Harnoss et al. however did not demonstrate sex predominance.2
The most common morphologic variant encountered was suprasplenic fusion (Figure 1). This is in keeping with the existing literature.2-4 Ishigami et al. did not evaluate the morphology of fusion based on a relationship with the splenic vein, instead classified it based on the pancreatic duct drainage. In PAP, the main pancreatic duct (MPD) can course either posterior to the PV (retroportal) [Type I] or anterior (anteportal) to it [Type III].5,8 Rarely it can be associated with pancreas divisum where one duct has a retroportal route and the accessory duct courses anterior to the PV [Type II]. These ductal and parenchymal features were combined in the classification proposed by Joseph et al.8 Though CT scan is primarily adequate to diagnose this anomaly, MRI helps to delineate the ductal anatomy and type this anomaly better.9 Lath et al. had reported an unusual ring-like configuration of the MPD on magnetic resonance cholangiopancreatography (MRCP) in a case of PAP, which they called the pancreatic duct ring sign.6 We did not evaluate the ductal anatomy in our study.
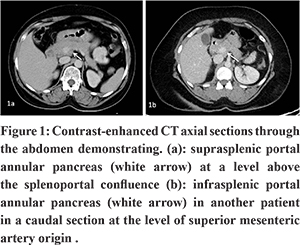
Though an incidentally detected anomaly in the majority of patients, PAP can be mistaken for a locally advanced focal lesion of the head / uncinate process of the pancreas encircling the vessels.6 This can especially happen when this anomaly is seen in patients with chronic pancreatitis where the rind of parenchyma appears hypertrophic in comparison to the remaining atrophic gland and hypo-attenuating as a sequel of previous inflammation as was seen in one of the cases (not in this study period) we had encountered in our practice (Figure 2). Correct identification of the anomaly will help prevent a major catastrophe when surgical drainage is attempted in chronic pancreatitis.
Furthermore, recognition of this variant is of paramount importance in patients planned for pancreaticoduodenectomy3. During the division of the pancreas at the neck in cases of PAP, two raw surfaces are created, and two ducts are divided. If not carefully dealt with, it can result in a postoperative pancreatic fistula. If the presence of this anomaly is known pre-operatively, the surgical technique can be modified accordingly.10,11 The resection line can be shifted to the left of the original resection line to pass through the body of pancreas, thus creating a single raw surface for anastomosis and reducing the risk of a postoperative fistula.10,11
Our study showed that radiologists did not specifically identify and report this entity in many cases. Our review of radiology reports revealed that the variant was mentioned in only one out of the 25 cases. The radiologists involved in reporting these cases were part of a heterogeneous group with varying levels of experience. It can easily be overlooked if one is not aware of the condition or does not look for it specifically. Many of the CT scans in the study group were not done for pancreaticobiliary pathologies. It is difficult to assess how many were overlooked and in how many cases the radiologist chose not to mention it as it was not relevant to the case. This could have contributed to the underdiagnosis of this anomaly in our series. Radiologists and surgeons need to familiarise themselves with this condition to avoid untoward consequences.
The limitation of this study was that the various subtypes of PAP were not assessed as it was a CT based study. MRI with MRCP may help delineate the ductal anatomy, which has considerable surgical significance.
Conclusion
Portal annular pancreas had a prevalence of 2.5% with a female preponderance in our series. This entity can be easily overlooked, and increased awareness is required to improve detection rates and thereby avoid problems during pancreatic surgery.
References
- Ishigami K, Tajima T, Nishie A, Asayama Y, Kakihara D, Nakayama T, et al. The prevalence of circumportal pancreas as shown by multidetector-row computed tomography. Insights Imaging. 2011 Apr 18;2(4):409–14.
- Harnoss JM, Harnoss JC, Diener MK, Contin P, Ulrich AB, Büchler MW, et al. Portal annular pancreas: a systematic review of a clinical challenge. Pancreas. 2014 Oct;43(7):981–6.
- Karasaki H, Mizukami Y, Ishizaki A, Goto J, Yoshikawa D, Kino S, et al. Portal annular pancreas, a notable pancreatic malformation: frequency, morphology, and implications for pancreatic surgery. Surgery. 2009 Sep;146(3):515–8.
- Yilmaz E, Celik A. Circumportal pancreas: prevalence, subtypes and vascular variations of 55 patients. SurgRadiol Anat. 2018 Jan 27;1–7.
- Sugiura Y, Shima S, Yonekawa H, Yoshizumi Y, Ohtsuka H, Ogata T. The hypertrophic uncinate process of the pancreas wrapping the superior mesenteric vein and artery —A case report—. Jpn J Surg. 1987 May 1;17(3):182–5.
- Lath CO, Agrawal DS, Timins ME, Wein MM. Portal annular pancreas: the pancreatic duct ring sign on MRCP. Radiol Case Rep. 2015 Dec;10(4):42–5.
- Arora A, Velayutham P, Rajesh S, Patidar Y, Mukund A, Bharathy KGS. Circumportal pancreas: a clinicoradiological and embryological review. SurgRadiolAnat SRA. 2014 May;36(4):311–9.
- Joseph P, Raju RS, Vyas FL, Eapen A, Sitaram V. Portal annular pancreas. A rare variant and a new classification. JOP J Pancreas. 2010 Sep 6;11(5):453–5.
- Patidar Y, Arora A, Mukund A, Dev A. Portal Annular Pancreas: An Under-Reported Pancreatic Anomaly. J Surg Tech Case Rep. 2012;4(2):140–1.
- Muto J, Mano Y, Harada N, Uchiyama H, Yoshizumi T, Taketomi A, et al. Additional resection of the pancreas body prevents postoperative pancreas fistula in patients with portal annular pancreas who undergo pancreaticoduodenectomy. Case Rep Gastroenterol. 2012 Jan;6(1):131–4.
- Kobayashi S, Honda G, Kurata M, Okuda Y, Tsuruta K. Pancreaticoduodenectomy in portal annular pancreas: report of a case. Surg Today. 2013 Aug;43(8):926–9.