|
Onelkis Feliciano1, Rosabel Falcon1, Amilcar Duquesne2, Osmel Fleitas1, Tatiana Almaguer1, Oderay Gutierrez1, Armando Orellana3, Virginia Capo4, Rafael Llanes1 1Center for Research, Diagnostic and Reference, “Pedro Kouri” Tropical Medicine Institute (IPK), Havana, Cuba. 2Microbiology Department, Fructuoso Rodriguez Hospital, Havana; Cuba. 3Endoscopy Department. Primary Healthcare Institution ¨19 de abril¨, Havana, Cuba. 4Histopathological Department, IPK, Havana, Cuba.
Corresponding Author:
MSc. Onelkis Feliciano Email: onelkisfs@infomed.sld.cu.
Abstract
Background: Helicobacter pylori infection is associated with the development of gastroduodenal diseases. Aim: This study was planned to assess the relationship between H. pylori-infection and bacterial antigen recognition in Cuban patients. Methods: One hundred-five subjects (7-82 years old) with gastroduodenal symptoms were enrolled. Collected sera were evaluated by Helicoblot 2.1 and the antigen seroprevalence growth by age decade was calculated. Results: Sixty-one (58.1%) subjects were considered infected with H. pylori by biopsy. The accuracy of Helicoblot 2.1 showed good sensitivity and specificity. VacA antigen seroreactivity was found to be significantly high in infected patients (P=0.00). The number of recognized proteins in H. pylori-infected patients from 40-61 years old was 2.5-fold superior compared to the youngest (5-17 years old) (P=0.04). Intestinal metaplasia and dysplasia were highly prevalent in the 40-50 age group. Conclusion: VacA protein can be considered a marker of H.pylori infection in Cuban population. In addition, a high frequency of premalignant lesions associated to H. pylori infection was observed in this study to suggest the establishment of early Cuban surveillance strategies.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|1928 48uep6bbph|2000F98CTab_Articles|Fulltext Introduction
Helicobacter pylori (H. pylori) is a Gram negative bacterium. Infection by H. pylori is mostly acquired in childhood and its persistence may lead to development of several gastroduodenal disease1. The microorganism is the major causative agent for non-ulcer dyspepsia; however, only a limited number of infected people develop peptic ulcer (PU) or gastric carcinoma (GC)2. The outcome is conditioned to the host response, enviromental factors and the H. pylori strain involved. These strain variations have been extensively studied. Putative factors like VacA and CagA proteins are considered crucial in the development of gastroduodenal diseases in Western countries1. Cuban H. pylori strains have shown a high prevalence of the main virulence factors. This is especially seen in elderly patients in whom multiple genotypes and more virulent types have been observed3. Nevertheless, the gastric cancer incidence is low (age-standardised incidence and mortality rate in both sexes of 5.9; http://globocan.iarc.fr). This fact suggests the importance of identifying serological markers in patients with high risk of developing severe gastroduodenal disease. International guidelines recommend the combined use of endoscopy with biopsy and histopathologic analysis for high risk patients and the development of biomarkers for premalignant lesions. Thi scan reduce the risk of gastric cancer in Latin America4. In Cuba, the diagnosis of H. pylori is made at the primary health care level using invasive and costly techniques5. Serological methods which are less expensive can be used for extensive population screening. The serological detection of the type of colonizing strains of an individual allows us to classify them into ahigh or low risk category. A diagnostic test that identifies sensitive markers of infection would be of great benefit to discriminate between peptic ulcer (PU) and gastric cancer (GC) H. pylori related diseases. Previous studies have used serology, in particular Helicoblot 2.1 (HB 2.1) for protein recognition and to differentiate patients with severe or mild gastroduodenal disease6,7. In Cuba there are no reports investigating H. pylori antibody response specific to virulence proteins in symptomatic individuals. The aim of the study was to identify the anti-H. pylori antibody protein recognition patterns using HB 2.1 in Cuban patients.
Methods
The study population included consecutive individuals with dyspeptic symtoms who had been referred for endoscopy in two Primary Healthcare Institutions in Havana City: ¨19 de abril¨and Pedro Borrás Hospital, from October to December 2014. Patients who had received antibiotic therapy, bismuth preparations, proton pump inhibitors or H2-blockers within the previous month were excluded. A total of 105 patients (range 7-82 years, median age of 44.5; 43 male and 62 female) were included in the study. Of these, 39 were children and 66 adults. All patients including children’s parents/guardians provided a written informed consent for participation in the study. Demographic data of each patient was collected. The protocol was approved by the Ethical Review Committee of the IPK (CEI-IPK 41-15).
Biopsy specimens, blood sample collection and immunoblot assay
During endoscopy four gastric biopsy specimens from the antrum were taken for culture, non-commercial rapid urease test (RUT)5 and histological examination following the updated Sydney criteria8. Patients were considered infected by H. pylori when culture was positive or, in the case of a negative culture, they had both a positive histological examination and a positive RUT. In contrast, when all of these were negative, patients were considered not to be infected by H. pylori. Blood samples (5 mL) were taken from each patient included in the study at the time of upper GI endoscopy and the separated sera were stored at -200C for Western blot evaluation. The Western blot qualitative assay was performed using the Helicoblot 2.1 kit (Genelabs Diagnostics, Singapore) as per following the manufacturer´s instructions. The nitrocelulose membrane strips of this kit contain a bacterial lysate plus H. pylori recombinant proteins (VacA, CagA, Urease B, HspA, 37kDa, 35kDa, Urease A, 19.5kDa and current infection marker: CIM). Densitometry (GS-800 Calibrate Densitometer; BioRad Laboratories, USA) was performed using Quantity One® software (BioRad) to determine the presence of inmunoreactive bands.
Statistical Analysis
The Chi-square test was used to compare the frequency of specific antigens between H. pylori infected and non-infected patients. The antigen seroprevalence increases with each decade of age. The trend of change in seropositivity with age group was estimated with the Mann-Whitney U test. The analyses were performed with GraphPad Prism version 5.01 for Windows (GraphPad Sofware San Diego California, USA). Statistical significance was defined as a P value less than 0.05.
Results
According to the gold standard tissue-based methods, 61 (24 male and 37 female) of 105 patients (58.1%) were H. pylori positive. The serological test HB 2.1 yielded a positive result in 54 of the infected patients (88.5%) and in four of the 44 non-infected patients (12.9%).The sensitivity, specificity, positive and negative predictive values for detection of H. pylori infection were 88.5 %, 90.9%, 93.1% and 85.1%, respectively. The demographic and clinical characteristics of patients (gender, age group and clinical form) shown no significant statistical differences) (Table 1).
The antibody to VacA protein was significantly increased in H. pylori-infected patients (P=0.00; OR: 3.3, 95% CI: 1.48-7.49). The values of seroreactivity against others antigens were similar in both groups (P>0.05) (Table 2).
Among H. pylori-infected patients, 40 had gastritis, 3 had peptic ulcer disease and 18 had premalignant gastric lesions (atrophic gastritis in 1, dysplasia in 2 and intestinal metaplasia in 15). There where no differences in seroreactivity to H. pylori antigens among patients having premalignant gastric diseases and gastritis (P>0.05) (Table 3). Atrophic gastritis was present in 5.6% of the subjects in the age groups of 18-28 and 29-39. Intestinal metaplasia and dysplasia were highly prevalent in the age group of 40-50. Premalignant gastric lesions were not observed in patients under 18 years of age (Figure 1).
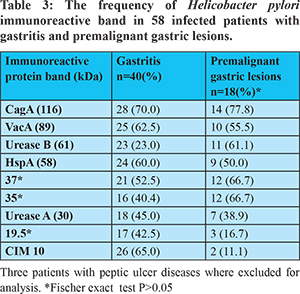
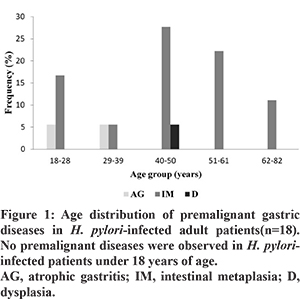
The number of recognised proteins among H. pylori-infected patients was also studied. It was observed that there was a 2.5-fold increase in the number of antigens recognized: five antigens were recognized in the middle age group (range: 40-50 years of age) but only two were recognized in the youngest age group (range: 5-17 years) (P=0.03). A similar trend was observed in the older groups of patients (51-61 and 62-82 years old) (P<0.05). The trend showed increase in the number of antigens with each decade of age (P=0.04) (Figure 2). The CagA protein recognition frequency increased with each decade of age (51.6, 50, 100, 83.3, 83.3 and 100%) whereas the frequency of VacA (51.6. 50, 50, 58.3, 66.7 and 50%) was constant over the time.
Discussion
Serological tests have been used worldwide in the last few years for assessing prevalence of H. pylori9. In particular, the immunoblot assay kit can investigate the possible relationship between clinical forms of the disease and the presence of specific H. pylori antigens. Previous studies have evaluated the accuracy of HB 2.1 commercial assay kit6,7. In the present study, the efficacy of HB 2.1 in the detection of H. pylori infection was assessed against gold standard tissue-based methods with good sensitivity and specificity. However, the estimation of negative cases was slightly lower than the estimation of positive cases, which could be due to cross-reactive antigens7. These results are similar to those reported previously6. The prevalence of H. pylori infection in the current study was similar to a previous report conducted in Cuba5, as well as in other developing countries6 but higher than in industrialised countries (Netherlands: 31.7% and Japan: 44.3%)10,11. The prevalence is influenced by the differences in socioeconomic conditions between societies12. The evaluation of multiple antibodies against specific H. pylori proteins allows us to estimate both: bacterial factors and host response. The results of the present study showed similar immunoreactive patterns between infected and non-infected patients with the exception of the VacA protein. The latter protein is an important virulence marker that plays a role in H. pylori colonization13. The colonisation process stimulates a humoral response with antibody production; such a response might be inefficient as shown by bacterial presence in chronically infected patients. The similar pattern of immunoreactive bands in the two groups of patients may be due to similarities in H. pylori antigens with the antigens present in enteric bacteria such as Campylobacter spp. In addition, H. pylori infection generates antibodies within approximately one month14. However, this serological response may persist after eradication; thus, the use of serological response as a predictive marker of gastrointestinal disease is controversial. The prevalence of antibodies to CagA and VacA found in patients with premalignant gastric lesions was similar to that in patients with gastritis. These antigens play a major role in pathogenicity and have been shown to be significantly related to gastroduodenal diseases in Western countries15. The lack of existence of an association between bacterial virulence factors and outcomes in the Cuban population has been reported by others authors16,17. The present study conducted in two primary healthcare institutions identified 18 patients with premalignant lesions. The finding of atrophic gastritis and intestinal metaplasia among young groups of adults was interesting and alarming at the same time, taking into account that the latter is a point of no return as proposed by Correa18. Besides, according to previous reports gastric cancer development depends on the severity of premalignant lesions19. Surveillance of patients with premalignant lesions such as atrophic gastritis and intestinal metaplasia could offer an opportunity for treatment for dysplastic lesions and gastric cancer in its early stages20. The number of proteins recognized by antibodies of H. pylori-infected patients was seen to increase with each decade. Similar results were obtained in a study conducted in Germany using multiplex serology in which antigen-specific seroreactivity increased with age21. Qualitatively, the increase in antibody reactivity with aging might also reflect high immunogenicity of bacterial antigens in individuals infected with multiple H. pylori strains. This pattern might be more prevalent in older age groups. The presence of multiple H. pylori strains (genotypes) colonising the gastric mucosa of Cuban patients and its association with age more than 40 years has been observed previously3. The current investigation has several limitations. First, samples were taken from small representative assessments. Second, patients with gastric cancer were not included. Nevertheless, the inclusion of individuals from a wide age range permits the determination of variation in humoral responses to H. pylori. This may have important biological implications that merit further evaluation. Further studies including a larger sample size balanced for each diagnosis will be necessary to better understand the association between virulence factors and clinical outcomes in Cuban H. pylori-infected patients. In summary, our results show that VacA protein is an important H. pylori infection marker for local usage. The present study showed preliminar results that will be taken in consideration to establish Cuban surveillance strategies addressed to high risk population.
References - Dunne C, Dolan B, Clyne M. Factors that mediate colonization of the human stomach by Helicobacter pylori. World J Gastroenterol 2014; 20: 5610-5624.
- De Luca A, Iaquinto G. Helicobacter pylori and gastric diseases: a dangerous association. Cancer Lett 2004; 213:1–10.
- Feliciano O, Gutierrez O, Valdes L, Fragoso T, Calderin AM, Valdes AE, Llanes R. Prevalence of Helicobacter pylori vacA, cagA, and iceA genotypes in Cuban patients with upper gastrointestinal diseases. Biomed Res Int. 2015;2015:753710.
- Flores-Luna L, Camorlinga-Ponce M, Hernandez-Suarez G, Kasamatsu E, Martínez ME, Murillo R, et al. The utility of serologic tests as biomarkers for Helicobacter pylori-associated precancerous lesions and gastric cancer varies between Latin American countries. Cancer Causes Control. 2013;24:241-8.
- Llanes R, Feliciano O, Guzmán D, Gutiérrez O, Valdés L, Llop A, et al. Use of a single biopsy specimen for diagnosing Helicobacter pylori infection by culture and two different PCR methods: report from Cuba. Trop Gastroenterol 2010;31:111-2.
- Vilaichone R, Mahachai V, Shiota SUchida T, Ratanachu-ek T, Tshering L, et al. Extremely high prevalence of Helicobacter pylori infection in Bhutan. World J Gastroenterol. 2013;19:2806-10.
- Chomvarin C, Ottiwet O, Hahnvajanawong C, Intapan PM., Wongwajana S. Seroreactivity to specific antigens of Helicobacter pylori infection is associatedwith an increased risk of the dyspeptic gastrointestinal diseases. Int J Infect Dis. 2009;13:647-54
- Malfertheiner P, Megraud F, O´Morain CA, Atherton J, Axon AT, Bazzoli F,et al.Management of Helicobacter pylori infection-the Maastricht IV/Florence Consensus Report. Gut 2012;61:646-64.
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterol. 2017;153:420-429.
- Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19:105-10
- van Blankenstein M, van Vuuren AJ, Looman CW, Ouwendijk M, Kuipers EJ.The prevalence of Helicobacter pylori infection in the Netherlands. Scand J Gastroenterol 2013;48:794-800.
- Salih, BA. Helicobacter pylori Infection in Developing Countries: The Burden for How Long? Saudi J Gastroenterol. 2009;15:201-7.
- Wang YK., Kuo, FC, Liu CJ,Wu MC, Shih HY, Wang SS, et al. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21:11221-35.
- Utsch C, Haas R. VacA’s Induction of VacA-Containing Vacuoles (VCVs) and Their Immunomodulatory Activities on Human T Cells. Toxins (Basel). 2016;8(6).
- Ki MR, Hwang M, Kim AY, Lee EM, Lee EJ, Lee MM, et al. Role of vacuolating cytotoxin VacA and cytotoxin-associated antigen CagA of Helicobacter pylori in the progression of gastric cancer. Mol Cell Biochem. 2014;396:23-32.
- Torres LE, Melian K., Moreno A et al., Prevalence of vacA, cagA and babA2 genes in Cuban Helicobacter pylori isolates. World J Gastroenterol. 2009; 2:204-210.
- Ortiz D, Guariglia V, Avila M et al., “Helicobacter pylori cagA and vacA genotypes in Cuban andVenezuelan populations. Mem Inst Oswal Cruz. 2010; 3:331-335.
- Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40:490-6.
- Lahner E, Bernardini G, Santucci A, Annibale B. Helicobacter pylori immunoproteomics in gastric cancer and gastritis of the carcinoma phenotype. Expert Rev Proteomics 2010;7:239-48.
- den Hoed CM, Holster IL, Capelle LG, de Vries AC, den Hartog B, Ter Borg F, et al. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy. 2013;45:249-56.
- Michel A, Pawlita M, Boeing H, Gissmann L, Waterboer T. Helicobacter pylori antibody patterns in Germany: a cross-sectional population study. Gut Pathog. 2014;6:10.
|