48uep6bbphidcol2|ID
48uep6bbphidvals|1925
48uep6bbph|2000F98CTab_Articles|Fulltext
Introduction
Gastric varices occur in 5 to 33% of patients with portal hypertension,1 accounting for 20% of cases of variceal haemorrhage.2 Bleeding can be torrential with a mortality rate as high as 45%.2 The factors predicting gastric variceal bleeding include: the size of the varix, presence of red signs and functional status of liver.3 The management of gastric varices is technically more challenging compared to esophageal varices, considering their larger size, deeper location, and position that demands retroversion of the endoscope for endotherapy. The therapeutic modalities include endoscopic interventions namely glue therapy and EUS-guided coil-embolization; radiological interventions, namely transjugular intrahepatic porto-systemic shunt, percutaneous transhepatic obliteration and balloon-occluded retrograde trans-venous obliteration; and rarely surgery.4
N-butyl-cyanoacrylate (NBC) glue injection is one of the well-established modalities to prevent and control gastric variceal bleeding, practised widely in countries like Germany, Italy, India, and Canada.5 In the United States, though not approved by the FDA in the treatment of gastric varices, it is used off-label.6,7 Despite being practised worldwide, the procedure of glue therapy for gastric varices lacks technical standardization.8
The principle of glue therapy is that on injection into the varix, it rapidly polymerises and hardens, thereby obturating the varix. Excellent immediate haemostasis rates (>90%) have been documented in studies.9
But there are certain potential life-threatening procedure-related adverse events such as bleeding, systemic glue-embolization, intravariceal needle embedment and sepsis.10 The incidence of immediate and early post-procedural bleed is around 6.8%.11 Such bleeding can at times occur from the puncture-site immediately upon withdrawal of the needle from the varix, due to its incomplete obturation. This can occur due to 1) usage of an incompatible flushing liquid resulting in premature glue solidification within the catheter before glue delivery, 2) usage of insufficient volume of flushing liquid due to non-availability of information on the dead space of the injection catheter, resulting in delivery of an inadequate quantum of glue into the varix, and 3) premature withdrawal of the needle from the varix before adequate variceal obturation, due to the lack of information on the solidification time of the glue. Delayed withdrawal of the needle from the varix after glue injection, on the other hand, can result in intra-variceal needle embedment. Therefore, the parameters of paramount importance in avoiding immediate puncture-site bleeding and intra-variceal needle embedment are 1) the proper choice of flushing liquid to prefill the catheter and push the glue from the catheter into the varix, 2) calculation of dead space of the injection catheter in use, and 3) information about the ‘solidification time’ of the glue in use, dictating the ‘minimum needle-indwelling time’ within the varix. Since several injection catheters and NBC glues are marketed, the knowledge of each of their dead space and solidification times respectively assumes significance.
As data on the ideal flushing liquid, the dead space of different injection catheters and solidification times of various glues are lacking, we conducted three in vitro studies (studies 1-3) in our hospital research laboratory in July 2015, aimed at analysing these technical determinants, to ensure safe and effective glue therapy without the adverse events of immediate puncture-site bleeding and intravariceal needle embedment. We have further correlated these observations in vivo, in a clinical study (study 4), where the incidence of immediate puncture-site bleeding and needle embedment was studied while the above-mentioned technical determinants were in practice. All the studies were carried out with the approval of the ethics committee and institutional review board.
Study 1
Background and Aim: An ideal flushing liquid is one which on contact with glue, does not trigger its premature solidification within the injection catheter, before it is delivered into the varix. Different liquid media such as distilled water12, normal saline13,7 and 5% dextrose14 have been used and reported in literature, for priming the catheter and for flushing the glue from the catheter into the varix. The compatibility of these liquids with glue needs to be assessed for standardisation of the procedure, since glue is vulnerable to premature intra-catheter solidification when an incompatible flushing liquid is used. This study aims to identify the ideal flushing liquid for pre-filling the catheter and flushing the glue into the varix.
Methods: Ten millilitre samples of the three test solutions namely solution A (5% dextrose), B (distilled water), and C (normal saline - NS) were taken in three petri dishes. NBC glue - Gesika (Reevax Pharma, India) was taken in an insulin syringe. A quantum of 1 ml of glue was added to the above three solutions in petri dishes, to identify incompatibility. The changes of incompatibility visible to the naked eye, in the form of development of a superficial filmy layer over the test solution, were documented by two independent observers blinded for the identity of the test solutions. The procedure was repeated with two other NBC glues namely, Endocryl (Samarth Pharma, India) and Histoacryl (Braun Melsungen AG, Germany), and the results were tabulated (Table 1).
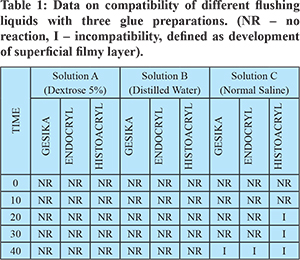
Results: In solution C (normal saline), incompatibility was observed within 40 seconds for all the three glues, whereas no changes were noted with solution A (5% dextrose) or solution B (distilled water) (Figure 1). The results showed that 5% dextrose and distilled water were compatible flushing liquids. Normal saline was concluded incompatible.
Study 2
Background and Aim: Dead space is the luminal volume of the injection catheter. Flushing liquid of a volume equal to the dead space of the catheter should be used in order to drive the glue completely out of the catheter into the varix. If the volume of flushing liquid falls short of the dead space, a part of the glue would remain within the catheter, resulting in inadequate delivery of the glue and consequently, incomplete variceal obturation, which may result in immediate puncture-site bleeding. On the other hand, usage of a volume larger than the dead space of the catheter carries the risk of glue embolization.3 Hence it is imperative to know the exact dead space of the catheter prior to glue therapy. Western reports have recommended a volume of 0.8 ml of flushing liquid for this purpose.8 However, no data is available on the dead space of commercially available injection catheters. Such information would ensure the usage of the correct volume of flushing liquid. This study aims to estimate the deadspace of three injection catheters marketed in India.
Methods: Three 21G injection catheters from three manufacturers, namely Innovative Therapeutics, Blue Neem Medical Devices and Biorad Medisys Pvt. Ltd. were studied. Using a 2 ml syringe, distilled water was injected slowly into each catheter until a tiny drop emerged out of the needle. The dead-space was estimated by subtracting the residual volume left in the syringe, from the initial volume of 2 ml.
Results: The dead space was estimated to be 1.4 ml for catheters from Innovative Therapeutics and Biorad Medisys Pvt. Ltd, and 1.6 ml for the catheter from Blue Neem Medical Devices. Hence, 1.4 to1.6 ml of flushing liquid would be needed to completely flush out the glue, instead of 0.8 ml recommended in certain reports.8 The use of just 0.8 ml of flushing liquid, can result in short delivery of glue, with part of it being left behind in the catheter. Hence an accurate estimation of deadspace of the catheter is essential.
Study 3
Background and Aim: The solidification times of different glues available commercially are variable. During glue injection, after injecting the glue into the varix, it is advisable to wait for a few seconds before withdrawing the needle from the varix. If the needle is withdrawn prematurely before the injected glue solidifies, it can result in bleeding from the puncture site. Allowing adequate time for the glue to solidify can prevent such a mishap. Hence the solidification time of the glue, which would dictate the minimum needle-indwelling time within the varix, assumes importance. As of now, no data on the solidification times of different glue is available in literature. This key information also lacks mention in product monographs. Information on the solidification time of the glue would serve as a guide to the endoscopist for determining the ideal ‘needle-indwelling time’ during the procedure. In this regard, the solidification times of three NBC glues availablein India were determined.
Methods: One ml of NBC glue from threeformulations, namely Glue A (Gesika - Reevax Pharma, India), Glue B (Endocryl- Samarth Pharma, India) and Glue C (Histoacryl -Braun Melsungen AG, Germany), were used. The time taken for complete solidification of glue, when added to Petri dishes containing 2 ml of un-clotted blood was measured using a stopwatch (Figure 2). The solidification time of glue was calculated as the time taken for the formation of a thick solid layer of glue over the blood sample. The procedure was repeated three times with each brand of glue and the mean solidification time was arrived at (Table 2).
Results: The time taken for solidification of glue (A) was 18.33 seconds, glue (B) was 24.67 seconds and glue (C) was 8 seconds.
Study 4
Aim: To study the incidence of immediate puncture-site bleed and intra-variceal needle embedment during glue therapy for gastric varices, when the above studied technical determinants namely, 1) compatible flushing medium, 2) optimal flushing volume equivalent to the dead space of the catheter, and 3) adequate needle-indwelling time corresponding to the glue solidification time, were put into practice.
Methods: From the observations made from our vitro studies, it has been our institutional protocol to measure the dead space of the injection catheter beforehand, to use a compatible flushing solution during the procedure and to observe a needle-indwelling time, according to the glue used. We have been using 21 G needle catheter from Innovative Therapeutics (dead space - 1.4 ml), 5% Dextrose as the flushing medium and NBC glue from Gesika, in all our glue injection procedures. Also, it has been our practice to observe a minimum needle-indwelling time of 20 seconds, (dictated by the glue solidification time determined in our in vitro study) in all our procedures.
In this study, records from our data base of glue injection procedures done between January 2016 to September 2017 were analyzed retrospectively. All patients with gastric varices who had undergone glue injection therapy at Medindia Hospitals, Chennai, India, during this period were included in the study. Those patients with uncorrected coagulopathy (INR > 1.5) and thrombocytopenia (platelet count < 50,000 cells/ml), were excluded from the analysis. All glue injection procedures were done as per standard Institutional protocol, as mentioned above. The incidence of immediate puncture-site bleeding and intra-variceal needle embedment was analysed from records. Immediate puncture-site bleeding was defined as active spurting that occurred upon removal of the needle catheter from the varix after glue injection, necessitating further glue injection. Intra-variceal needle embedment was defined as the cementing of the needle into the solidified glue within the varix, resulting in either detachment of the needle from the catheter or laceration of the varix, upon forceful removal of the catheter.
Results: Twenty-two patients underwent glue injection for gastric varicesin a total of 58 glue injection procedures. The average volume of glue injected per patient was 2.6 ml. In 15 patients, the procedure was done following variceal bleed as secondary prophylaxis, and in sevenpatients as primary prophylaxis in ‘high-risk’ varices. There was no immediate puncture-site bleeding or intra-variceal needle embedment following any of the procedures, when all the three technical determinants were put into practice.
Discussion
Glue therapy for the treatment of gastric varices was first described in 1986 by Soehendra N et al.15 Though glue therapy has been in existence for over 30 years, the exact technique has not been standardized.10,16 The safety and effectiveness of the procedure depends on the adequate obturation of the varix upon complete delivery of glue from the catheter into the varix. Conventionally, a mixture of glue and lipiodol was used. Lipiodol delays polymerization of glue within the catheter. However, it has been incriminated in distant embolization.16 Glue without the use of lipiodol has been demonstrated to be safe and effective in terms of variceal obturation.17
The serious procedural adverse events of immediate puncture-site bleeding and intravariceal needle embedment were addressed in this study. The three technical determinants which play a role in these adverse events, namely the flushing liquid, the variability of the dead space of injection catheters and the variable solidification times of the different glues were studied.
The ideal flushing medium sans lipiodol to prefill the catheter and flush the glue has not been analyzed earlier. Using an incompatible flushing liquid would result in premature solidification of the glue within the catheter. In our in vitro study, dextrose and distilled water were found to be most suitable for pre-filling the catheter and for flushing the glue into the varix.
The dead space of the injection catheters varies from one manufacturer to another. In our study, we estimated the dead space of 21 G injection catheters from three manufacturers available in India. The dead space was estimated to be 1.4 ml for Innovative and Biorad systems and 1.6 ml for the Blue-Neem catheter. It should be emphasized that if only 0.8 ml of flushing liquid is used, as reported in the Western report8, injecting 1 ml of glue followed by 0.8 ml of flushing solution would result in delivery of only 0.4 ml of glue into the varix,as 0.6 ml would be retained within the catheter, if the Innovative or Biorad systems injection catheters are used; and only 0.2 ml of glue would be delivered and 0.8 ml would remain within the catheter, if the Blue-Neemcatheter is used. This would consequently lead to incomplete obturation of the varix resulting in a high chance of immediate puncture-site bleeding. On the other hand, flushing the glue using a solution of a volume in excess of the dead-space, can increase the risk of distant embolism.3 Hence, estimating the exact volume of deadspace of the injection catheter, before the procedure, is of paramount importance.
The solidification time is variable for different glues available worldwide. During the procedure, after injecting the glue into the varix, the needle is withdrawn after an optimal period, to allow for the polymerization of glue. Premature withdrawal of needle before adequate solidification time the glue is fraught with the risk of immediate puncture-site bleed, which at times can be life-threatening. We had previously described and reported two signs (“catheter pull sign” and “red catheter sign”) to predict immediate puncture-site bleed.18 On the other hand, delayed withdrawal of the needle may result in the needle getting impacted into the solidified glue cast.19,20 In such scenarios, forcible withdrawal of the impacted needle can result in laceration of the varix. Therefore, knowing the optimal needle-indwelling time for the glue in usage is important. In the present study, we found that at least 8 to 25 seconds was needed for complete solidification, depending on the glue used. We suggest that observing a ‘minimum needle-indwelling time’ dictated by the in vitro glue solidification time will prevent the adverse events of immediate puncture-site bleeding and needle embedment. This has hitherto not been reported in literature.
The results of our in vitro studies were adopted in our procedure of glue injection. The fourth study was carried out retrospectively by analyzing the data on glue injection procedures during the study period. It demonstrated that observing the three technical determinants during the procedure was helpful in avoiding immediate puncture-site bleeding. The adverse event of intravariceal needle embedment reported in a few studies,19,20 was also not encountered in any of our cases. Observing a ‘minimum needle-indwelling time’ corresponding to the solidification time of the glue, appears to minimize the risk of both immediate puncture-site bleeding and needle embedment.
The small number of cases and absence of a control group are the limitations of the study. Larger prospective studies may be required to validate these observations.
Conclusions
The procedural factors intrinsic to the adverse event of immediate puncture-site bleeding in glue therapy for gastric varices are the usage of incompatible or inadequate volume of flushing medium to drive the glue and premature withdrawal of the injection catheter from the varix, before adequate solidification of the injected glue. Flushing media like normal saline are incompatible with glue causing premature glue solidification. We suggest the dead space of the injection catheter be estimated beforehand and factored in, since it is variable. And further, an adequate needle-indwelling time, based on the solidification time of the glue, is recommended to prevent immediate puncture-site bleeding and intra-variceal needle embedment. To the best of our knowledge, these three studies are the first to analyze, the technical determinants which address these adverse events. The results of these studies emphasise the usage of a compatible flushing liquid, and the need for awareness of the variability of the dead space of injection catheters and solidification times of different NBC glues. Our clinical study further validates the results of our in vitro studies.
References
- Garcia-Tsao G, Sanyal A, Grace N, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-38.
- Sarin S, Lahoti D, Saxena S, Murthy N, Makwana U. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16(6):1343-9.
- Kim T, Shijo H, Kokawa H, Tokumitsu H, Kubara K, Ota K et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25(2):307-12.
- Levy M, Wong Kee Song L. EUS-guided angiotherapy for gastric varices: coil, glue, and sticky issues. Gastrointestinal Endoscopy. 2013;78(5):722-5.
- D. Christodoulou, E. V. Tsianos, P. Kortan, N. Marcon. Gastric and ectopic varices – newer endoscopic options. Annals of Gastroenterology 20(2):95-109 •
- Bhat YM. Tissue Adhesives for Endoscopic Use. Gastroenterology & Hepatology. 2014;10(4):251-3.
- Bhat Y, Banerjee S, Barth B, Chauhan S, Gottlieb K, Konda V et al. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointestinal Endoscopy. 2013;78(2):209-15.
- Ang T, Seewald S, Soehendra N. Endotherapy of Gastric Fundal Varices: Intravariceal Injection of N-Butyl-2-Cyanoacrylate. Video Journal and Encyclopedia of GI Endoscopy. 2013;1(1):157-9.
- Belletrutti P, Romagnuolo J, Hilsden R, Chen F, Kaplan B, Love J et al. Endoscopic Management of Gastric Varices: Efficacy and Outcomes of Gluing with N-Butyl-2-Cyanoacrylate in a North American Patient Population. Canadian Journal of Gastroenterology. 2008;22(11):931-6.
- Seewald S, Sriram P, Naga M, Fennerty M, Boyer J, Oberti F et al. Cyanoacrylate Glue in Gastric Variceal Bleeding. Endoscopy. 2002;34(11):926-32.
- Saraswat V, Verma A. Gluing Gastric Varices in 2012: Lessons Learnt Over 25 Years. Journal of Clinical and Experimental Hepatology. 2012;2(1):55-69.
- Mahmoudi N, Whittaker J. Glueing of Fundal Varices. Canadian Journal of Gastroenterology. 2006;20(11):691-3.
- Rivet C, Robles-Medranda C, Dumortier J, Gall C, Ponchon T, Lachaux A. Endoscopic treatment of gastroesophageal varices in young infants with cyanoacrylate glue: a pilot study. Gastrointestinal Endoscopy. 2009;69(6):1034-8.
- Moore C, Murphy K, Gailloud P. Improved distal distribution of n-butyl cyanoacrylate glue by simultaneous injection of dextrose 5% through the guiding catheter: technical note. Neuroradiology. 2006;48(5):327-32.
- Soehendra N, Nam V, Grimm H, Kempeneers I. Endoscopic Obliteration of Large Esophagogastric Varices with Bucrylate. Endoscopy. 1986;18(01):25-6.
- Seewald S, Ang T, Imazu H, Naga M, Omar S, Groth S et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos). Gastrointestinal Endoscopy. 2008;68(3):447-54.
- Kumar A, Singh S, Madan K, Garg P, Acharya S. Undiluted N-butyl cyanoacrylate is safe and effective for gastric variceal bleeding. Gastrointestinal Endoscopy. 2010;72(4):721-7
- Chandrasekar T, Menachery J, Gokul B, Murugesh M, Vivek Sandeep T. Novel predictors for immediate puncture site bleed during endoscopic glue injection for gastric varices without using lipiodol. Indian Journal of Gastroenterology. 2013;32(3):200-3.
- Bhasin D, Sharma B, Prasad H, Singh K. Endoscopic removal of sclerotherapy needle from gastric varix after N-butyl-2-cyanoacrylate injection. Gastrointestinal Endoscopy. 2000;51(4):497-8
- Lorenz A, Städtler N, Schulz H. Laser Disintegration of Cyanoacrylate Clot with Successful Endoscopic Removal of Sclerotherapy Needle from Gastric Varix. Endoscopy. 2002;34(8):670-2