48uep6bbphidcol2|ID
48uep6bbphidvals|1888
48uep6bbph|2000F98CTab_Articles|Fulltext
Tyrosinemia type 1 is caused by a defect in enzyme fumarylacetoacetase which is final enzyme of the pathway of the degradation of tyrosine. As a result of the metabolic block, toxic metabolites are formed including succinylacetone (SA), maleylacetoacetate and fumarylacetoacetate. These are responsible for severe disruption of intracellular metabolism of the liver and kidney. NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1, 3-cyclohexanedione) is the only drug which is used in management of tyrosinaemia type 11. It is a potent inhibitor of 4-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme that is upstream of fumarylacetoacetasehydrolase (FAH). As a result the flux through the pathway is markedly reduced and in most patients there is a rapid decrease in the concentrations of succinylacetone (SA), an increase in tyrosine and a clear clinical improvement. As NTBC has adverse effect like corneal opacity, leukopenia, thrombocytopenia, convulsions, there is a need to monitor the levels of NTBC in the blood and adjust dose accordingly. We present a child with tyrosinemia diagnosed in May 2012, started on NTBC in July 2012 and needed a dose adjustment based on serum NTBC levels as serum tyrosine was very elevated in January 2016.
Case Report
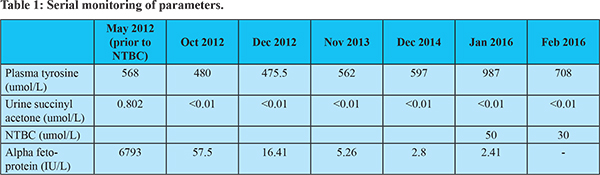
A 1 year 9 month old girl was diagnosed to have tyrosinemia in May 2012. She had hepatosplenomegaly with renal tubular acidosis (RTA), alpha fetoprotein (AFP) was 6793 IU/L and urine SA was elevated (0.802mmol/l, Control = 0.001). She has been started on tyrosine and phenylalanine low diet along with bicarbonate supplement and NTBC (1 mg/kg/day)from July 2012(Shah I, Shah F 2006). Her serial AFP and Urine SA as well as serum tyrosine levels aredepicted in Table 1. She had a first episode generalised tonic clonic convulsion in Feb 2014 along with fever and was diagnosed to have febrile convulsion. Subsequently, she had a left focal convulsion in June 2015 without fever. EEG showed abnormal focal slowing in the right occipital region. She was started on leviracetam and advised serum levels of NTBC. In January 2016, her NTBC levels were 50 umol/L (normal range 30 to 50 umol/L) and simultaneous serum tyrosine levels were 987 umol/l. Her dose of NTBC was decreased to 0.8 mg/kg/day. Repeat tyrosine in February 2016 was 708 umol/l and serum NTBC levels were 30 umol/L. She was advised same dose and regular follow-up. Her liver function tests, venous blood gases and hemogram continue to remain normal. Her milestones and growth are also appropriate for age.
Discussion
With the introduction of NTBC (nitisinone) the prognosis for thosewith tyrosinemia type 1 has improved greatly.Nitisinone treatment has significantly improved the outcomesof patients with tyrosinemia type I, while decreasing utilization of healthcare resources, liver transplants, and associated costs2.
As soon as the diagnosis of tyrosinemia 1 is confirmed, NTBC is started in adose of 1 mg/kg/d once a day as the half-life is 54 hrs3. A dose of 2 mg/kg/d should be given for 48 hoursfor those in acute severe liver failure. An alternativeapproach is to give all patients in liver failure, nitisinone ata dose of 2 mg/kg/d from the start and allow the dose tofall with growth to 1 mg/kg/d before increasing it. NTBCcan only be given orally (or by naso-gastric tube). It is imperative to do so quickly to prevent further liver and kidney damage and avoid potentially major complicationssuch as haemorrhage. The risk of long term complicationsis also reduced4. The response to nitisinone is usually rapid. Coagulationusually improves within 48 hours and all patients should respond within a week. Succinylacetone in the urine andblood should no longer be detectable after the first 24 hours. Nitisinone must be continued without interruption. If not continued may precipitate serious complications including acute liver failure, a neurological crisis4 or even hepatic malignant change. On the standard dose of 1 mg/kg/d, the plasma concentrationsin individuals that suppress SA are variable3,4. Adjustments of the dose based on plasma NTBC concentrations may be indicated. However the target NTBC concentrations in plasma are uncertain andvary from 30-50 µM5. Some prefer to maintain the concentration above 50 µM5. However lower doses of NTBC have been found to be effective8. In one case the serum nitisinoneconcentration was only maintained above 30 µmol/l8 with apparently good metabolic control. Whatever the dose, complete suppression of succinylacetone concentrationsis essential. Our patient was on 1 mg/kg/day dose of NTBC for 3 years 5 months. Then in January 2016 her plasma levels of NTBC were done as the serum tyrosine levels had increased and subsequently dose of NTBC was titrated to maintain at the lower limit of normal so that plasma tyrosine levels decrease. Also whether the seizure disorder in our patient was due to NTBC or a sequelae of febrile convulsion remains undetermined.
Not all centres monitor NTBC levels in patients. While metabolic control can be judged by SA levels/excretion in urine overdosing and compliance can only be judged by measuring NTBC levels. Monitoring of NTBC plasma levels is therefore useful and permits individual dosing. Thus, treatment costs and side effects can be minimized without hampering metabolic control.
In conclusion, monitoring of NTBC levels is essential in patients with tyrosinemia 1 to titrate the dose so that maximum effect can be achieved by minimising adverse effects of NTBC.
References
- Santra S, Baumann U. Experience of nitisinone for the pharmacological treatment of hereditary tyrosinaemia type 1. Expert OpinPharmacother. 2008; 9:1229–1236.
- Mariève Simoncelli, Johanne Samson, Jean-François Bussières, Jacques Lacroix, Marc Dorais, Renaldo Battista et al. Cost–Consequence Analysis of Nitisinone for Treatment of Tyrosinemia Type I. CJHP 2015;68,210-217.
- Schlune A, Thimm E, Herebian D, Spiekerkoetter U. Single dose NTBC treatment of hereditary tyrosinaemia type 1. J Inherited Metab Dis. 2012; 35:831–836.
- Schlump JU, Perot C, Ketteler K, Schiff M, Mayatepek E, Wendel U,Spiekerkoetter U. Severe neurological crisis in a patient with hereditarytyrosinaemia type I after interruption of NTBC treatment. J Inherit MetabDis. 2008, 31(Suppl 2):S223–S225.
- Holme E. Disorders of tyrosine degradation. In Physicians Guide to theTreatment and Follow-Up of Metabolic Diseases. Edited by Blau N, Leonard J,Hoffmann GF, Clarke JTR. Berlin: Springer. 2006:49–56.
- El-Karaksy H, Rashed M, El-Sayed R, El-Raziky M, El-Koofy N, El-Hawary M,Al-Dirbashi O. Clinical practice. NTBC therapy for yrosinaemia type 1:how much is enough? Eur J Pediatr. 2010; 169:689–693.