48uep6bbphidcol2|ID
48uep6bbphidvals|1866
48uep6bbph|2000F98CTab_Articles|Fulltext
Colorectal cancer is the third most commonly diagnosed cancer and the third leading cause of cancer death among adults.1 On the contrary, primary gastrointestinal malignancies constitute not more than 2% of all childhood tumors. Out of these colorectal (CR), malignancies in childhood are very rare and are second to hepatic malignancies in terms of incidence. The mortality from colorectal cancer in both men and women has declined in the last three decades, but the situation remains grim among the younger patients afflicted with CR malignancy.2 The literature with regards to CR cancers among adults is robust, but the same for childhood CR malignancy is scanty and understandably so because of its rarity among children. Through this review of our experience, we intended to project the issues that are peculiar to CR malignancies among the pediatric population.
Materials and Methods
This retrospective study was conducted in a pediatric surgical oncology clinic of a tertiary care pediatric surgery department. The study involved the extraction of pertinent data of all cases diagnosed with colorectal carcinoma between 2006 and 2014. The parameters that were noted include age, sex, symptoms, examination findings, specific investigations, the extent of the disease (stage), therapy received, outcome and the follow-up.
Results
A total of 1061 new childhood cancer cases were registered in the pediatric solid organ oncology clinic of the Institute during the above period, and only five patients (0.3%) had CR carcinoma. The median age of the patient was 11 years (range, 10-12 years). Male to female ratio was 1.5:1. None of them had any predisposing conditions or family history of colorectal malignancy.
The main symptom (n=4) was bleeding per rectum (PR). The other clinical features are described in Table 1. The duration of onset of symptoms prior to presentation ranged from 2 weeks to 1 year. On physical examination, one patient (case no 2) had a palpable abdominal lump whereas all others had a mass palpable on per rectal examination.
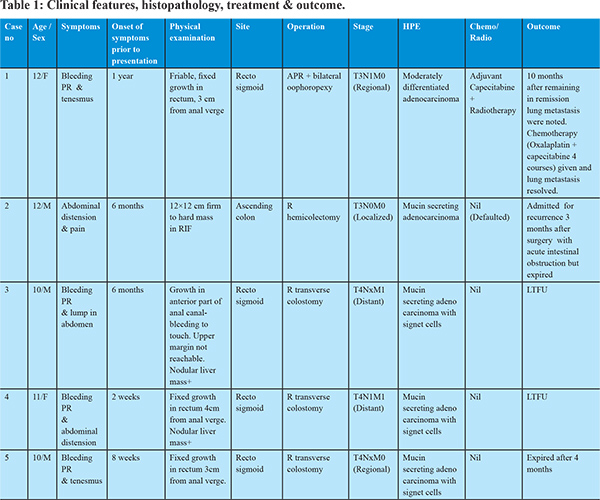
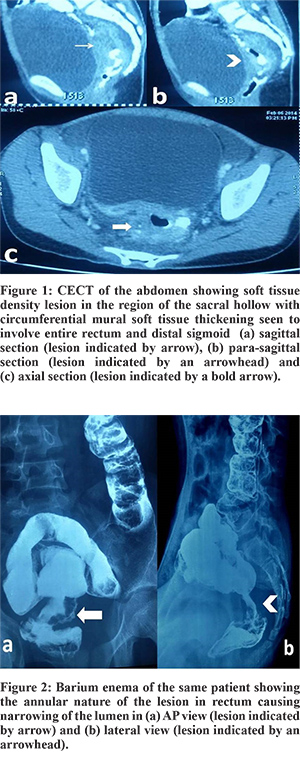
Following baseline investigations, all patients underwent a CECT scan, and all of them showed the involved segment of bowel with soft tissue mass effect (Figure 1). CEA was done in one patient but was not elevated. Barium enema was already done in one child before presentation to our center (Figure 2). Colonoscopy was done in 2 patients that corroborated with the CT findings and also enabled procurement of tissue for histopathological examination (HPE). The HPE revealed adenocarcinoma in all. Operation with curative intent was performed in two patients in the form of abdomino-perineal resection and right hemicolectomy (case no. 1 and 2). Two patients had metastatic disease at presentation (case no. 3 and 4), whereas one patient (case no. 5) had locally advanced disease (T4) but no metastasis. Proximal diversion (right transverse colostomy) was only performed in these three patients. Only one patient (case no. 1) completed postoperative concurrent chemoradiotherapy that included oral Capecitabine (850 mg/m2, five days a week) and radiotherapy (45 Gy in 25 fractions over five weeks).
Longest follow up in our series was 15 months seen in only one case (Case no. 1). Case no 2 followed up after three months after undergoing right hemicolectomy, but developed a local recurrence (defaulted to receive adjuvant therapy). Case no. 3 and 4 were lost to follow up after palliative proximal diversion while case no. 5 followed up after four months, but he had developed extensive intra-peritoneal dissemination and died.
Only one patient (Case no. 1) received curative surgical attempt followed by adjuvant therapy. After remaining in remission for ten months, bilateral lung metastases were picked up during surveillance. She received chemotherapy (CapeOX: Capecitabine 1000 mg/m2 bid on days 1–15 and Oxaliplatin 130 mg/m2 on D1 q3wk). After four courses she was reassessed, and it was noted that the lung metastasis had resolved. Subsequently, she completed two more courses of CapeOX and is currently under surveillance. Case no. 2 expired after three months with local recurrence, acute intestinal obstruction, pneumonia, and multi-organ failure.
Discussion
It is known that more than 90% of colorectal cancer cases occur in people aged 50 or older. Recent trends show that colorectal cancer is increasing among younger persons. In fact, in the United States, colorectal cancer is now one of the ten most commonly diagnosed cancers among men and women aged 20 to 49 years.3,4 In the first two decades of life, however, the involvement is not more than 1%. Because of its rarity in the pediatric population the diagnosis of CR carcinoma is generally not considered initially, and the disease often spreads extensively before the diagnosis is made.
The biological behavior of these tumors in the pediatric population is collated only from various case reports and case series. Management of these cases is also extrapolated from guidelines that are well established in adults but are nonexistent separately for children. Rao et al. from St. Jude’s hospital has described 30 pediatric patients treated for CR tumors in 1985.5 Yang et al. in 2008 reported a population-based analysis of 270 pediatric colorectal tumors and their outcomes and found that majority of them occurred on the right side of colon and usually prevalent among the teens.6 He emphasized the importance of complete surgical excision in achieving some form of survival. Pandey et al. from India presented their experience of 4 cases of rectal carcinoma and found a dismal outcome and emphasized per rectal examination for early identification of rectal malignancy in patients presenting with bleeding PR.7
There are quite a few peculiar points of CR malignancy in the pediatric population that sets them apart from those of the elderly. It is notable that compared to adults, the presentation of children with CR malignancy in an early stage is infrequent. This was also noted in our series, and can be because in children a symptom of blood in stools generally is attributed to benign conditions like polyps or a Meckel’s diverticulum. Recent onset constipation is often approached with differentials such as fissure-in-ano. Also, children with other symptoms like vague abdominal pain are suspected to suffer from worm infestations, and the underlying disease process progresses covertly to achieve untreatable proportions. This factor has important implications towards the outcome as CR cancer survival is highly dependent upon stage of disease at diagnosis, and typically ranges from a 90% 5-year survival rate for cancers detected at the localized stage; 70% for regional; to 10% for patients diagnosed for distant metastatic cancer among adults.8
Various predisposing conditions have been described for CR cancers in adults. They are inflammatory bowel disease, family history of CR Cancer, familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC), also called Lynch syndrome. Usually, approximately 5 to 10% of colorectal cancers are a consequence of recognized hereditary conditions.9 We did not have any patient in our series with either family history or predisposing syndromes. Similar observations were made by Pandey et al. in their series of 4 cases.7
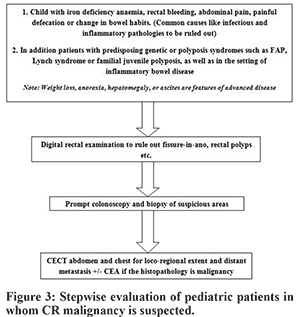
In the young almost the entire colon is equally affected by malignancy unlike in adults where only rectal and left sided lesions predominate.5 In our series, however, we saw 4 out of 5 cases arising from the recto-sigmoid region. Diagnosis is easy to miss unless clinical suspicion is high in the early stages. In our cases since the cases were advanced at presentation, so all of them had physical findings in the form of either abdominal lump (1/5 cases) or mass per rectum (4/5 cases). In early stages, various modalities that can help in clinching the diagnosis are colonoscopy, barium enema study, and CT scan. Colonoscopy has the advantage of procuring tissue for biopsy, and CT scan can elaborate the anatomical details including loco-regional as well as distant metastasis. Since there are no clear guidelines, we suggest evaluating pediatric patients for CR malignancy with even minor symptoms soon after common causes have been ruled out (Figure 3). This may ensure that the cases are at least picked up at an early stage. In our patients, CT scan was done in all however Barium enema, and colonoscopy was done only in one patient. Laboratory profiling of tumor marker of CEA was not done routinely, and only one case had undergone this test, and the values were normal. The role of CEA in adults is well established, but it’s role in children is unclear. Vastyan reported 7 cases of pediatric colorectal carcinoma, and none of them had elevated CEA levels.10 Rao et al. also noted this finding that in children the CEA levels usually remains normal with either disease progression or in the presence of residual disease even in Duke’s C or D stage.5
An unfortunate fact about the pediatric CR malignancy is the type of histology that is distinct from that commonly found in adults. In the majority of the pediatric CR cases, the histology is that of poorly differentiated mucin-producing adenocarcinoma, and this leads to a poorer overall outcome. Mucinous adenocarcinoma comprises only 5% of the adult CR malignancies. The mucinous component results in aggressive behavior with easy penetration into tissue planes leading to widespread involvement of neighbouring structures. It is also known to blunt the immune mechanisms against the neoplastic cells as the mucin masks the recognition of the relevant antigens.11 In a recently published large cohort of patients with CR malignancies of the National Cancer Data Base from 1998 to 2011, Poles et al. compared the disease pattern among pediatric patients with that of adults. They found that CR carcinoma had a more aggressive tumor histology and behavior in children, particularly in rectal cancer.12 In our group, 4 out of 5 cases had mucinous adenocarcinoma.
The treatment strategy is mainly based on operative extirpation. However, curative surgery is difficult to offer as nearly 80% of childhood, and adolescent patients have Dukes stage C or D involvement at the time of diagnosis as a consequence of the delay in diagnosis.5,13 The principle of operation includes resection of the appropriate section of the involved large bowel and its lymph nodes. Curative operation is found to be possible in only 40% of pediatric CR malignancies.5,14 Similarly, we could offer curative surgery to only two patients, and palliative surgery was done in others.
Despite the poor overall outlook, a pediatric patient diagnosed with colorectal carcinoma has improved survival in comparison with prior reports. Currently, there are no treatment algorithms exclusively for pediatric CR malignancies. The knowledge from adult literature is generally adapted to manage pediatric CR cases. Since 2005, Capecitabine (a prodrug of 5-Fluorouracil) has been approved by FDA for management of CR malignancy in adjuvant setting as either monotherapy or combination with other drugs like Oxaliplatin and also with concurrent radiotherapy.15 Out of four cases in our series, only one patient received concurrent adjuvant chemoradiation and remained in remission for ten months. She however later developed pulmonary metastasis and then she was administered chemotherapy based on Capecitabine and Oxaliplatin. Complete response was seen after four courses and has received two more courses subsequently and is currently under surveillance.
CR malignancy should be considered as one of the rare childhood cancer. Survival can only possibly improve if they are identified early. Collaborative clinical research, DNA sequencing and molecular biology must be explored to characterize the biological behavior of pediatric CR malignancies further. This would also provide data regarding the efficacy of the adjuvant treatment modalities that are currently being utilized from guidelines for the adult population.
To conclude, the survival in pediatric CR malignancies is low, and the prognosis is poor. Adjuvant therapy in the form of radiation and chemotherapy has the potential to improve survival despite having an aggressive biological behavior as compared to adults. Pediatric surgeons should start giving importance to seemingly minor symptoms and to consider CR malignancy as a differential diagnosis for these symptoms to diagnose CR malignancy at an early stage.
References
- American Cancer Society (2011). Colorectal Cancer Facts and Figures 2011–2013. Atlanta: American Cancer Society.
- Boyle P, Ferlay J. Mortality and survival in breast and colorectal cancer. Nat ClinPract Oncol. 2005;2:424–425.
- O’Connell JB, Maggard MA, Liu JH et al. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866–872.
- O’Connell J B, Maggard M A, Livingston E H et al. Colorectal cancer in the young. Am J Surg. 2004;187:343–348.
- Rao BN, Pratt CB, Fleming ID et al. Colon carcinoma in children and adolescents. A review of 30 cases. Cancer. 1985;55:1322-6.
- Yang R, Cheung MC, Zhuge Y et al. Primary solid tumors of the colon and rectum in the pediatric patient: a review of 270 cases. J Surg Res. 2010; 161:209-216.
- Pandey A, Gangopadhyay A, Sharma S et al. Pediatric carcinoma of rectum--Varanasi experience. Indian J Cancer. 2008;45:119-122.
- Ries L AG, Melbert D, Krapcho M et al. SEER cancer statistics review, 1975–2005. Bethesda, MD: 2008.
- Jackson-Thompson J, Ahmed F, German RR et al. Descriptive epidemiology of colorectal cancer in the United States, 1998-2001. Cancer. 2006;107:1103–1111.
- Vastyan AM, Walker J, Pintér AB et al. Colorectal carcinoma in children and adolescents--a report of seven cases. Eur J Pediatr Surg. 2001;11:338-341.
- Karnak I, Ciftci AO, Senocak ME et al. Colorectal carcinoma in children. J Pediatr Surg. 1999;34:1499-1504.
- Poles GC, Clark DE, Mayo SW et al. Colorectal carcinoma in pediatric patients: A comparison with adult tumors, treatment and outcomes from the National Cancer Database. J Ped Surg. 2015. DOI: http://dx.doi.org/10.1016/j.jpedsurg.2015.11.005 (Article in press)
- Sahoo SP, Gangopadhyay AN, Gupta DK et al.Carcinoma of the rectum in children. Pediatr Surg Int. 1996;11:418-420.
- Brown RA, Rode H, Millar AJ et al. Colorectal carcinoma in children. J Pediatr Surg. 1992 ;27:919-921.
- Hirsch BR, Zafar SY. Capecitabine in the management of colorectal cancer. Cancer Management and Research 2011;3: 79–89.