48uep6bbphidcol2|ID
48uep6bbphidvals|1864
48uep6bbph|2000F98CTab_Articles|Fulltext
Celiac Disease (CD) is a chronic immune-mediated multi-systemic disease that can affect any organ. Extra-intestinal manifestations are seen in up to 30% of patients with CD.1 Modest elevation of serum aminotransferase levels is common in untreated CD, occurring in 15%-55% of patients.2 Cirrhosis in patients with CD is described only in case reports.3,4 Cirrhosis is often associated with increased morbidity/mortality, decrease in health related quality of life and substantial health care costs.5 Without a liver transplantation survival is very poor; prevention of development of cirrhosis and its progression are considered standard of care. Therefore recognizing CD in the setting of cirrhosis is essential in order to institute a GFD to potentially prevent further morbidity and mortality. Data is still lacking on correlation of CD with cirrhosis and the response to GFD in cirrhotics. Here we present six cases of CD with cirrhosis of liver where we have studied duration of disease, mode of presentation and effects of GFD on liver disease. Diagnosis of cirrhosis was based on clinical, biochemical, endoscopic and imaging findings. Patients with histological evidence of cirrhosis were also included. The diagnosis of alcoholic cirrhosis was made on the basis of history of any form of daily alcohol consumption of >80g/dl in men and >40g/dl in women for 10yrs. Diagnosis of CD was done as per European Society of Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) guidelines (score of 4 or more was considered diagnostic of CD). IgA anti endomysial antibodies (EMA) was done using ELISA kit named D-TEK from Belgium. Normal range is 0-25 units/ml. Value of 25 units/ml or more is considered positive.
Case 1
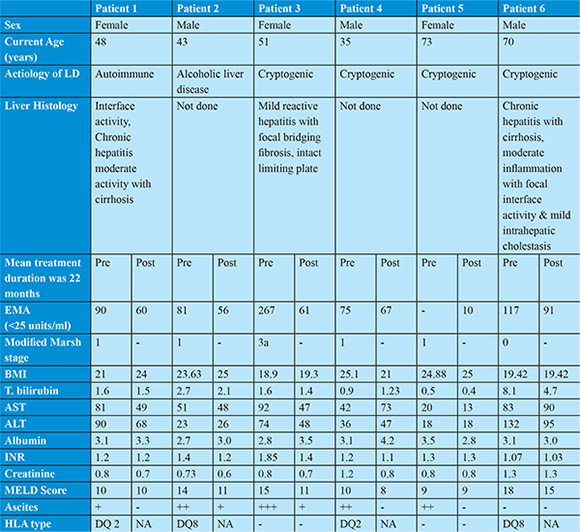
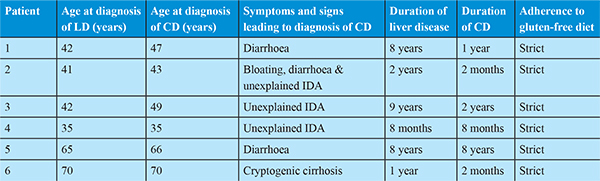
A 48 years old female with presented 8 years back with complain of painless progressive symmetrical distension of abdomen and painless swelling of both lower limbs since 1 month. She had no co morbidities and no addictions. After all laboratory investigations, upper gastrointestinal endoscopy (small oesophageal varices) and ultrasound of abdomen (USG abdomen) (moderate ascites, nodular liver surface and mild splenomegaly) and liver biopsy (moderate interface activity, chronic hepatitis with cirrhosis); she was diagnosed to have autoimmune hepatitis with decompensated cirrhosis of liver and was treated with tapering doses of steroids and azathioprine. One year later she presented with complains of small bowel type diarrhoea for 2 months. On investigations she had iron deficiency anaemia, USG abdomen showed moderate ascites and UGIscopy showed small oesophageal varices, duodenum was normal but biopsy was taken from second part of duodenum. Histopathology showed >40 intraepithelial lymphocytes/100enterocytes with normal crypts and normal villi. Colonoscopy was normal. EMAwas elevated more than 3 times the upper limit of normal. HLA typing showed HLA DQ2 positive. Patient was started on GFD. On one year of strict GFD patient‘s symptoms improved, ascites resolved diuretics were stopped and there was no worsening in liver functions.
Case 2
A 43 years old male presented 2 years back with history of painless swelling of both lower limbs feet since 2 years. He had history of significant alcohol intake (180ml/day) for 20 years. He had history of systemic hypertension since 1 year. UGI scopy showed no varices with mild portal hypertensive gastropathy and USG abdomen showed minimal ascites, shrunken and nodular liver and mild splenomegaly. He was diagnosed to have alcohol related decompensated cirrhosis of liver after ruling out all other causes of cirrhosis. Over a period of 2 years he was abstinent from alcohol and liver disease was stable with no fresh decompensation. Now he presented with dyspnoea on exertion since 2 months. On investigating he was found to have severe iron deficiency anaemia (IDA) (Hemoglobin-6gm/dl). There was no history of malena or hematochezia. UGI scopy and colonoscopy were normal. Duodenal biopsy from 2nd part of duodenum was done. Histopathology showed >40 intraepithelial lymphocytes/100enterocytes with normal crypts and normal villi. Capsule endoscopy was normal. USG abdomen showed moderate ascites. For further evaluation of IDA, EMA was done which was elevated > 3 times the upper limit of normal. HLA typing showed HLA DQ8 positive. Patient was started on GFD and intravenous iron replacement was done. One year on GFD patient’s hemoglobin improved, his diuretics requirement diminished and there was no worsening in liver functions.
Case 3
A 51 years old female presented 9 years back with history of two episodes of hemetemesis, distension of abdomen and fever since 1 month. After all laboratory investigations, UGI scopy (large oesophageal varices and endoscopic variceal band ligation done) and USG abdomen (moderate ascites, small nodular liver and mild splenomegaly and liver biopsy (mild reactive hepatitis with focal bridging fibrosis, intact limiting plate); she was diagnosed to have decompensated cryptogenic cirrhosis of liver. On regular follow up 2 years later she was found to have IDA on routine investigations. On upper GI scopy showed small oesophageal varices without bleeding, duodenum was normal but biopsy was taken from second part of duodenum. Histopathology showed mild villous atrophy and >40 intraepithelial lymphocytes/100enterocytes. Colonoscopy was normal. On further work up for IDA, EMA was done, which was elevated > 10 times the upper limit of normal. HLA typing could not be done. Patient was started on GFD. On 2 years of GFD her hemoglobin improved, his diuretics requirement diminished and there was no worsening in liver functions.
Case 4
A 35 years old male presented 10 months back with history of malena since 1 month and distension of abdomen since 10 days. He was diagnosed to have decompensated cirrhosis of liver with ascites and IDA. Patient refused to give consent for liver biopsy. Even after correction of serum iron levelpatient persistently had IDA. Stool for occult blood was negative on three occasions. In view of unknown cause of cirrhosis and persistent IDA he was screened for celiac disease. His EMA was elevated 3 times the upper limit of normal. His duodenal biopsy showed >40 intraepithelial lymphocytes/100enterocytes with normal crypt and villi. He was HLA DQ2 positive. Patient was started on GFD. After 8 months of GFD patient showed significant improvement.
Case 5
A 73 years old female presented with history of small bowel diarrhoea for 2 months. On laboratory investigations she had IDA, thrombocytopenia liver function test normal serum bilirubin, showed low serum albumin, albumin globulin ratio reversal and ALT/AST ratio reversal, serum B12, serum calcium and vitamin D levels were normal. EMA antibodies were more than 10 times elevated. Duodenal biopsy showed crypt hyperplasia with >40 intraepithelial lymphocytes per 100 enterocytes (Marsh stage 2). USG abdomen showed small and nodular liver, mild splenomegaly and no ascites. She was started on GFD and steroids. She presented to us 5 months back for routine follow up. She was on GFD and prednisolone 10 mg per day since last 8 years. She had no decompensation in last 8 years and her MELD score remained same.
Case 6
A 70 years old male presented 2 months back with history of jaundice and mild itching all over the body since 1 year. He had no history of clay coloured stool, distension of abdomen, altered sensorium, hemetemesis and malena. He was diagnosed to have cryptogenic cirrhosis. Patient was screened for celiac disease in view of cryptogenic cirrhosis. EMA levels were more than 4 times elevated. Duodenal biopsy showed normal duodenal mucosa. HLA type DQ8 was positive. Patient was started on GFD. Patient improved, his bilirubin levels decreased by nearly 50%. His MELD score decreased from 18 to 15 in 2 months.
Discussion
CD is increasingly reported in a variety of liver disorders. Its prevalence in cirrhotic patients was not established, until a recent study showed that CD affects 2.5% of cirrhotic patients.1 This is more than twice the prevalence in the general population.6 Not all patients with severe liver failure and CD have apparent symptoms compatible with CD, suggesting that the CD related liver involvement was not necessarily a complication of malabsorption.7 Ventura et al showed that prolonged exposure to gluten in patients with CD contributes to the development of other autoimmune diseases such as diabetes mellitus or autoimmune thyroid disorders.8 Hepatic involvement is usually considered to be mild, but some studies findings suggest that in some cases it may eventually lead to an end-stage liver disorder.1
Three pathophysiologic mechanisms are now proposed on how CD affects the liver.1 First hypothesis is; due to gene linkages with other autoimmune disorders, CD may occur in association with other autoimmune liver diseases. Second hypothesis is; the proximity of the liver to the small intestines allows both organs to share important physiologic features. Third hypothesis is; malnutrition and bacterial overgrowth are other mechanisms reported to induce hepatic damage in patients with CD.
There is one earlier anecdotal report in which a GFD was shown to reverse severe liver failure in two patients with CD with PBC and liver transplantation was no longer needed.9 Fleming et al also suggest that GFD had a favourable effect on the liver due to the finding that all 4 surviving patients with CD showed normalization of their liver tests after excluding gluten from their diet, our study also confirms this as 5 out of 6 of our patients showed improvement in terms of decrease in ascites, decrease in transaminases levels, decrease in bilirubin levels and improvement in MELD score in 2 patients. This further supports screening for CD in cirrhosis.
Amongst our patients all had high titres of EMA, but 4 were in modified marsh stage 1, one in stage 0 (i.e normal small bowel biopsy). Prior report had shown that higher titers of EMA can diagnose CD with in patients with normal small bowel biopsy.10 HLA typing was done in 4 /6 patients, HLA DQ 2 and 8 were seen in two patients each. Patient with normal small bowel biopsy (modified marsh stage 0) had HLA type DQ 8. Five of our patients had mild changes on small bowel biopsy (modified Marsh 0-2), but the diagnosis CD was confirmed as they met the ESPGHAN criteria for diagnosis of CD. There is no clear explanation for the mild changes on small bowel biopsy.
Modes of presentation of CD in our patients were IDA and Diarrhoea. CD can occur coincidentally with other liver disorders and screening may be warranted during the evaluation of patients with cirrhosis. Abnormal EMA and high anti TTG levels can be used to diagnose CD in cirrhosis. Through this case series we would like to emphasize that CD should be rigorously investigated in all patients with AIH or hepatitis of unknown aetiology. In some cases, early detection and treatment of CD with GFD may prevent progression to end-stage liver failure.
References
- Wakim-Fleming J, Pagadala MR, McCullough AJ, Lopez R, Bennett AE, Barnes DS, Carey WD. Prevalence of celiac disease in cirrhosis and outcome of cirrhosis on a gluten free diet: a prospective study. J Hepatol. 2014; 61: 558-63.
- Kaukinen K, Halme L, Collin P, Farkkila M, Maki M, Vehmanen P, et al. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology 2002;122:881–8.
- Al-Hussaini A, Basheer A, Czaja AJ. Liver failure unmasks celiac disease in a child. Ann Hepatol. 2013;12:501–5.
- Roumeliotis N, Hosking M, Guttman O. Celiac disease and cardiomyopathy in an adolescent with occult cirrhosis. Paediatr Child Health. 2012;17:437–9.
- Korpimäki S, Kaukinen K, Collin P, Haapala AM, Holm P, Laurila K, Kurppa K,Saavalainen P, Haimila K, Partanen J, Mäki M, Lähdeaho ML. Gluten-sensitive hypertransaminasemia in celiac disease: an infrequent and often subclinical finding. Am J Gastroenterol. 2011 Sep;106(9):1689-96.
- Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 2003;163:286–92.
- Ratziu V, Nourani M, Poynard T. Discussion on celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure.Gastroenterology. 2002;123:2158-9.
- Ventura A, Magazzu G, Greco L. Duration of exposure to glutenand risk for autoimmune disorders in patients with celiac disease. Gastroenterology 1999;117:297–303.
- Neuberger J. PBC and the gut: the villi atrophy, the plot thickens. Gut. 1999;44: 594–5.
- Wakim-Fleming J, Pagadala MR, Lemyre MS, Lopez R, Kumaravel A, CareyWD, et al. Diagnosis of celiac disease in adults based on serology test results,without small-bowel biopsy. Clin Gastroenterol Hepatol 2013;11:511–516.