48uep6bbphidcol2|ID
48uep6bbphidvals|1848
48uep6bbph|2000F98CTab_Articles|Fulltext
Parasitic enteric infections are a serious health burden in developing countries, though they appear to be generally uncommon in developed countries.1 The significant reduction of routine exposure to parasitic worms, such as helminths, in highly industrialized countries has been proposed as a cause of the increased incidence of ulcerative colitis (UC) and Crohn’s disease (CD). This supposition, known as the“inflammatory bowel disease (IBD) hygiene hypothesis”, postulates that the reduced exposure to infectious agents has limited the immune-system response, causing damage to important regulatory immunological circuits.2 Intestinal parasites are predominantly small bowel pathogens but the colon can also be involved.1
As a consequence of the IBD hygiene hypothesis, and after considering the low prevalence of intestinal parasites and related complications in developed,3 CD is considered one of the most common causes of bowel obstruction and/or perforation in young patients living in Western countries. Here we report a case of ileal perforation, previously attributed to Crohn’s disease, that subsequently turned out to be a complication of a helminthic infection.
Clinical Case
In 2016, a 48-year-old Italian male patient attended our IBD Unit because of a previous diagnosis of CD. His past medical history was not relevant for any disease. In 2015, he was admitted to the Emergency Department of another hospital because of an acute (onset within the last 24 hours) and worsening abdominal pain, associated with nausea and vomiting. Laboratory tests showed leukocytosis (WBC 20.000), hyperfibrinogenaemia and C reactive protein (CRP) 8 times the upper limit of normal (u.l.n.). As a consequence, the patient underwent ultrasonography evaluation, which showed free liquid in the abdomen and a plain X-ray abdomen that showed gas under the right dome of diaphragm. A computed tomography revealed the presence of free gas in the peritoneal cavity and a swelling of the terminal ileum, cecum and peritoneum. The patient was taken up for emergency surgery. Intra-operatively, the surgeon found a large mass which included the terminal ileum, cecum and omentum, with signs of ileal perforation. This mass was resected “en bloc” (20 cm of ileum and 15 cm of colon) and an end-to-side ileocolic anastomosis was constructed.
Histological examination of the surgical section revealed the presence of acute and chronic inflammatory infiltrate, with mucosal and submucosal erosion, while the serosa and mesenteric adipose tissue appeared diffusely infiltrated by lymphogranulocytes and granulation tissue with areas of fibrosis. Therefore, the patient received a diagnosis of CD and was started on prophylactic therapy with 5-ASA (2.4 g/day).
Two months after surgery, the patient presented abdominal pain, non-bloody diarrhea (4-5 bowel movements per day) and diffuse cutaneous itch. He underwent serological evaluation and fecal exams, which appeared normal, and started therapy with metronidazole without benefit.
In 2016 (after 6 months of surgery) the patient presented to our IBD Unit.
On admission, he reported diarrhea (4 bowel movements per day), cramping abdominal pain and a chronic itch treated by anti-H1 agents. Abdominal palpation revealed abdominal pain without mass. Serological tests were normal, except for CRP (5 times the u.l.n.); in addition, fecal tests and fecal calprotectin were normal. Abdominal and bowel sonography was performed: no bowel wall thickening and/or bowel complications were evident.
Considering the previous diagnosis of CD, the patient underwent an ileo-colonoscopy: the colonic mucosa, the ileocolic anastomosis and the pre-anastomotic ileum showed no signs of inflammation. Considering the clinical history of our patient and the outcome of diagnostic re-evaluation, we critically revised his diagnosis. We requested a histological revision of the surgical sections made in 2015. The histological second-look revealed ischemic phenomena of the epithelium, with superficial ulcerations. Only in two samples, the pathologist observed transmural inflammatory infiltrate with consensual peritonitis. Moreover and surprisingly, some helminthic cytoskeletal-like organoids structures were observed, without recognition of complete structures. No histological signs of CD were found (Figure 1).
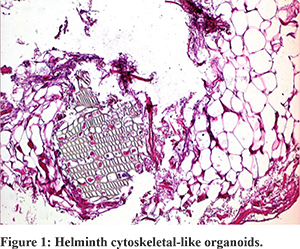
Thus, after that the diagnosis of CD was rejected, the patient was re-evaluated for the presence of parasitic infections. Despite the fact that fecal examinations and anti-Anisakis antibodies were normal, a course of therapy with albendazole was suggested.
Interestingly, after 1 month from the therapy, the patient reported resolution of the abdominal pain, diarrhea and itch; laboratory tests showed the normalization of CRP.
In addition, after a further 6-months period of follow-up, the patient became asymptomatic.
Discussion
Crohn’s disease represents one of the most frequent causes of obstruction and perforation of the small bowel in developed countries. Here we reported a case of ileal perforation, primarily attributed to CD, but subsequently ascribed to helminth infection.
In effect, small bowel obstruction and perforation are rare but severe complications of helminth infection and often occur with the migration of helminths into the peritoneal cavity, resulting in peritonitis. The exact incidence of primary helminthic perforation of the small bowel is not known. The proposed mechanism of this complication is direct pressure and irritation of the bowel wall by impacted helminth masses, leading to ulceration, necrosis, and perforation. Lytic secretions may also play a role.1
A review of literature revealed other previous cases of bowel perforation due to helminth infection but, as expected, almost all of them originated in developing countries, where the infection is endemic and, contextually, the prevalence of IBD is very low.
In the case we reported, a native European patient, without risk factors for a helminthic infection (such as travel to endemic areas or a compromised immune system), received a diagnosis of CD before reaching the correct diagnosis.
In a recent study in South Italy, Belli et al3 found a prevalence of intestinal parasitosis of about 10% in more than 1400 native patients: in the vast majority of cases it was a protozoan, largely represented by facultative pathogens, while helminths were found in less than 1% of cases and were represented by only a few species, such as Ascarides, Taenia, and E. vermicularis. This result was significantly associated with a history of recent travel in endemic areas. The authors concluded that in developed countries not all parasites are extinct, may be because many of them are zoonotic agents. Thus, in the routine protocol for the evaluation of patients with bowel symptoms, especially those with a history of travel to an at-risk area, a parasite search should always be considered, even though pre-test probability is very low.
The prevalence of intestinal parasitosis is increasing in industrialized countries probably in association with the globalization of the food supply, immigration/adoption from endemic regions, and travels through the same areas. The relatively mild or non-specific symptoms, the long incubation periods, and the unavailability or the inadequacy of the laboratory methods for diagnosis contribute to underestimating the prevalence of these infections in industrialized regions. Furthermore, European control strategies are limited and only concern a few pathogens, and most of the parasitic diseases are subjected to notification in only some countries. Moreover, physician awareness about these diseases is frequently poor; as a result, they are often neglected.4
In our patient, fecal tests were always normal, delaying the diagnostic suspect of helminth infection; moreover, the first histological evaluation did not reveal signs of helminths, probably because of the very low prevalence of microscopic features in a non-endemic area. As a consequence, the low pre-test probability of ileal perforation due to helminth infection in a patient belonging to a developed country without risk factors for intestinal parasitosis had led to the diagnosis of CD.
As mentioned before, the prevalence of intestinal parasitosis is high in developing countries; at the same time, almost the totality of cases reporting helminthic complications (obstruction and/or perforation) originate in developing countries, where the infection is endemic.5 Further more, a histological second-look could be useful to exclude or confirm a previous CD diagnosis when clinical and laboratory features appear controversial.
In conclusion, helminth infection, although considered a parasitic disease of tropical countries, is not absent in Europe and other Western countries and may indeed be responsible for cases of bowel obstruction and perforation. Clinicians should be conscious of the possibility, though rare, of intestinal parasitoses, which are able to induce severe complication, mimicking Crohn’s disease.
References
- Hechenbleikner EM, McQuade JA. Parasitic Colitis. Clin Colon Rectal Surg2015;28:79–86. ?doi:10.1055/s-0035-1547335
- Castiglione F, Diaferia M, Morace F, Labianca O, Meucci C et al. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: a case-control, multi-centre, prospective study in Southern Italy. J Crohns Colitis. 2012;6:324-9. doi: 10.1016/j.crohns.2011.09.003.
- Belli A, Coppola MG, Petrullo L, Lettieri G, Palumbo C, Dell’Isola C, Smeraglia R, Triassi M, Spada E, Amoroso P. The current spectrum and prevalence of intestinal parasitosis in Campania (region of southern Italy) and their relationship with migration from endemic countries. International Journal of Infectious Diseases 2014;29:42–47. doi: 10.1016/j.ijid.2014.04.021
- Calderaro A, Montecchini S, Rossi S, Gorrini C, De Conto F, Medici MC, Chezzi C, Arcangeletti MC. Intestinal parasitoses in a tertiary-care hospital located in a non-endemic setting during 2006–2010. BMC Infectious Diseases 2014, 14:264. doi:10.1186/1471-2334-14-264?
- Singh UC, Kumar A, Srivastava A, Patel B, Shukla VK, Gupta SK. Small bowel stricture and perforation: an unusual presentation of Fasciolopsisbuski. Trop Gastroenterol. 2011;32:320-2.