48uep6bbphidcol2|ID
48uep6bbphidvals|1846
48uep6bbph|2000F98CTab_Articles|Fulltext
Primary hepatic lymphomas are a rare neoplasm of obscure etiology. Recent case reports in Hepatitis C virus (HCV) positive patients have postulated possible role of HCV in their pathogenesis. Most cases present with a solitary large hepatic mass in a non-cirrhotic liver. We herein report a case of diffuse large B cell lymphoma (DLBCL) with germinal center phenotype presenting as multiple > 30 liver nodules in a cirrhotic liver.
Case Report
We report a case of 53-year-old female who is a known seropositive for HCV since 10 years on medical management. She presented with complaints of jaundice for 6 months. She developed distension of abdomen and pedal edema 3 months back. No history of ascitic taps in the past. There is no history of upper GI bleed, spontaneous bacterial peritonitis, hepatorenal syndrome or hepatic encephalopathy. There was no past history suggestive of diabetes mellitus, hypertension, chronic obstructive pulmonary disease or asthma. She was admitted and evaluated for liver transplant. Her International Normalized Ratio (INR) at admission was 3.04 which later increased to 3.61. She was managed with fresh frozen plasma transfusions. She had ascites and an ascitic tap was performed under USG guidance with around 5 litres of fluid drained. Her ascitic fluid TLC count was 150/mm3. She was managed with intravenous antibiotics. Her Hemogram revealed Haemoglobin 11.2 mg/dl, PCV 34.4%, TLC 10,200/mm3, DLC N77/L15/M7/E1, platelet count 68,000/mm3. Liver function tests showed bilirubin total/direct 6.26/4.71mg/dl, AST 94IU/L, ALT 69IU/L, ALP 162IU/L, GGT 55IU/L, protein 5.8 g/dl, albumin 1.7g/dl, INR 3.04 and Fibrinogen 171 mg/dl. Renal function test revealed BUN 16mg/dl, creatinine 1.0 mg/dl. Hepatitis B surface antigen was found to be positive and Hepatitis B core antibody was reactive (9.850). Her anti-HCV was positive and HCV RNA levels were 14500 IU/ml. HCV Genotype was 3. She tested negative for HIV type 1 and 2. Her AFP, CEA, CA-19-9 levels were 6.55ng/ml, 2.66ng/ml, 46.56ng/ml respectively. CT Angio liver performed revealed chronic liver disease. There were multiple hypodense SOLs of variable sizes in both the lobes of liver which do not show obvious arterial enhancement. Most of the lesions do not show appreciable enhancement in Porto venous phase, however, few of the lesions show mild enhancement in Porto venous phase. Features suggest the possibility of regenerative nodules.
She was operated within a week. During surgery, her native liver was shrunken and nodular.
Liver Explant-Explant weighing 1.7 kg and measuring 18.5 x 14.5 x 8.0 cm. The liver is firm. The external surface was brownish nodular, margins were irregular. The liver is serially sectioned at 0.5 to 1 cm interval to show a tan brownish color diffusely multinodular cut surface, with nodules ranging from 0.2 to 0.5 cm in diameter. There were multiple distinct nodules (>30) in all lobes of liver with whitish fleshy cut surface varying in size from smallest 0.9 cm in diameter to largest 2.5 cm in diameter (Figure 1). Larger nodules showed central necrotic areas.
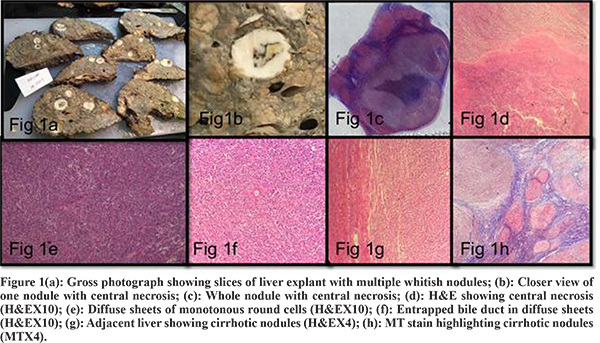
Multiple sections examined from different whitish fleshy nodules show a malignant round cell tumor composed of uniform intermediate to occasional large round cells in sheets having irregular vesicular nuclei and focally prominent nucleoli, entrapped bile ducts noted at the periphery of several nodules. Large nodules showed central areas of necrosis (Figure 1). Adjacent liver tissue showed multiple regenerating parenchymal nodules intervened by fibrous septae. Several foci of large cell change noted. Areas of spotty and confluent necrosis noted.
On immunohistochemistry, neoplastic cells were LCA, CD10, CD20 Positive and variable nuclear positivity for BCl6. Neoplastic cells were negative for Pan CK, CD 99, Bcl2, Mum 1, S100, CD 3, and cyclin D1. Ki67 index is 85-90 % (Figure 2). The case was diagnosed as Diffuse Large B cell lymphoma; germinal center phenotype with high Ki67 index. Cytogenetic testing done for c-myc, bcl2 were negative.
Thereafter, bone marrow aspiration and biopsy was done. Bone marrow aspiration showed Normoblastic and megaloblastic erythroid hyperplasia and Myeloid and megakaryocytic series within the normal limit with Lymphopenia. PET scan didn’t show any significant uptake except at ribs (probably due to trauma).
Thereby, a final diagnosis of primary hepatic lymphoma, diffuse large B cell type was rendered in an HCV and HBV related cirrhotic liver.
The patient was advised R-CHOP regimen in view of CD 20 positivity; however, she denied treatment and is well on a follow-up 6-month post-transplant.
Discussion
According to the criteria outlined by Caccamo et al, PHL is defined as a lymphoma with only liver involvement at presentation, while there is an absence of splenic, lymph node, peripheral blood, bone marrow or other tissue involvement for at least 6-month post-diagnosis.1
PHL are commonly of Diffuse large B cell type, followed by MALTomas, T cell lymphoma and Burkitt’s lymphoma.2
Etiological association of PHL has long been questioned and remained obscure. However recent reports of development of PHL in cases of Hepatitis C virus have postulated a possible association between HCV and clonal proliferation of lymphoma cells. 50% cases of PHL in a review by Kanta et al were positive for HCV RNA.2 A French group in a retrospective study on 31 lymphoma cases reported a high prevalence of HCV in PHL. Bronowicki et al.3 reported HCV infection in 6/28 cases of PHL. A possible aetiological link between chronic liver disease and primary hepatic lymphoma has also been postulated by Japenese group on a study on 51 cases of PHL.4 Our case also showed the development of PHL in HCV related cirrhotic liver.
Primary hepatic lymphoma most commonly affected middle-aged males.4 PHL causes nonspecific symptoms, right upper quadrant pain and rarely lymphoma-associated B symptoms, such as fever, night sweating, and weight loss. Our patient also did not have any symptom due to lymphoma. Patients with PHL typically have abnormal liver function tests, with an elevation of lactate dehydrogenase (LDH) and alkaline phosphatase. AFP levels are usually within normal limits.
Most cases of PHL present as solitary mass less commonly as diffuse lesions and rarely as multinodular lesions. Our case showed the presence of multiple >30 nodules in a cirrhotic liver. Ultrasound show classically hypo-echoic lesions relative to normal liver. The abdominal CT scan show hypo-attenuating masses, unenhanced or poorly enhanced after contrast as was seen in our case.
PHL requires a multimodality treatment and surgery, radiotherapy, and chemotherapy have been used alone or in combination for treatment.
For HCV-associated DLBCL, anthracycline-based chemotherapy [usually cyclophosphamide, hydroxydaunorubicin, vincristine, prednisolone (CHOP)] associated with rituximab (immuno-chemotherapy) is the standard of care.5Thus, rituximab and CHOP regimen followed by antiviral treatment may result in an improved clinical outcome in primary hepatic and systemic HCV-seropositive DLBCL cases.
PHL are rare extra-nodal lymphomas. Increasing case reports and reviews are highlighting the possible role of HCV in clonal expansion of lymphoma cells. Our study also highlighted the development of multinodular PHL in HCV related cirrhosis giving way for future molecular studies.
References
- Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986; 110:553–555
- Kikuma, K, Watanabe, J, Oshiro, Y, Shimogama, T, Honda Y, Okamura S, et al. Etiological factors in primary hepatic B-cell lymphoma. VirchowsArchiv, 2012: 460(4), 379–387
- Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, et al. Primary lymphoma of the liver. Hepatology. 2003;37:781–787.
- Higuchi T, Nomoto K, Mori H, Niikura H, Omine M, Sekiyama K, Yoshiba M, Fujita R. Case report: primary hepatic lymphoma associated with chronic liver disease. J Gastroenterol Hepatol. 1997 Mar;12(3):237-42.
- Kim JH, Kim HY, Kang I, Kim YB, Park CK, Yoo JY, Kim ST.A case of primary hepatic lymphoma with hepatitis C liver cirrhosis. Am J Gastroenterol. 2000 ; 95(9):2377-80.