NP Kamalesh, K Prakash, M Philip*, K Pramil, A Sylesh, P Shaji, GT Deepak, S Rohan
Dept of GI Surgery and Gastroenterology* PVS Memorial Hospital, Cochin, Kerala, India
Corresponding Author:
Dr. N P Kamalesh
Email: kamli6@yahoo.co.uk
48uep6bbphidcol2|ID 48uep6bbphidvals|1704 48uep6bbph|2000F98CTab_Articles|Fulltext Menetrier’s Disease is a rare form of hypertrophic gastropathy characterized by giant gastric rugal folds and associated with enteral protein loss and hypoalbuminemia. Until now only around 200 cases have been reported, and of these only fewer than 20 cases have been associated with gastric adenocarcinoma. Our case is unique due to its rarity: the incidental detection of adenocarcinoma in a patient first detected with Menetrier’s disease and the presence of characteristic clinicopathologic manifestations.
Case History
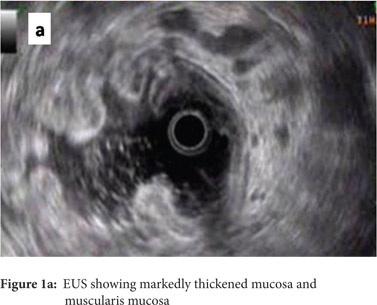
A 49 year old gentleman presented to our department with the history of loose stools, anorexia, weight loss and generalized malaise of 3 month’s duration. He had no other complaints. At presentation he was anemic and had bilateral pedal edema. His abdominal examination was unremarkable. His hemogram, liver function tests and renal parameters were essentially normal except for a low serum albumin of 2.0 mg/dl. An ultrasonogram abdomen showed diffuse wall thickening involving the fundus of the stomach, and upper gastrointestinal endoscopy revealed hypertrophic rugal folds involving the fundus and body of the stomach along with hypersecretion of mucus. Endosonography showed marked thickening of the mucosa and muscularis mucosa with normal appearing submucosa, muscularis propria and serosa (Figure 1a).
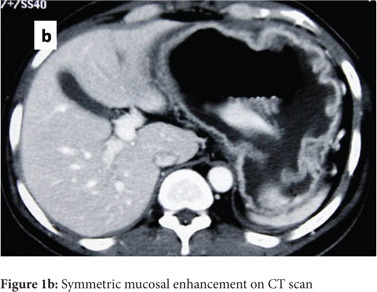
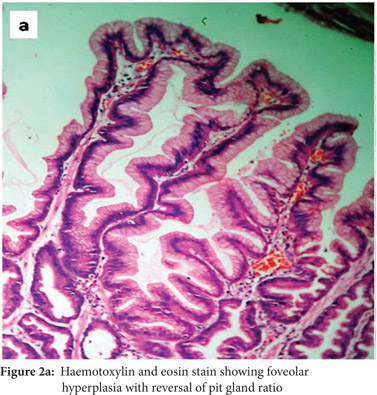
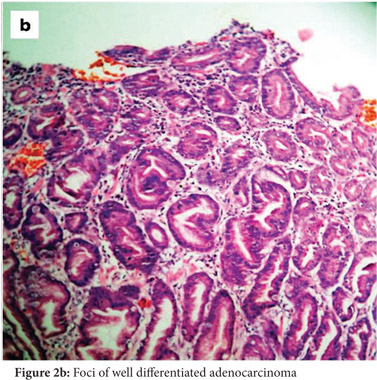
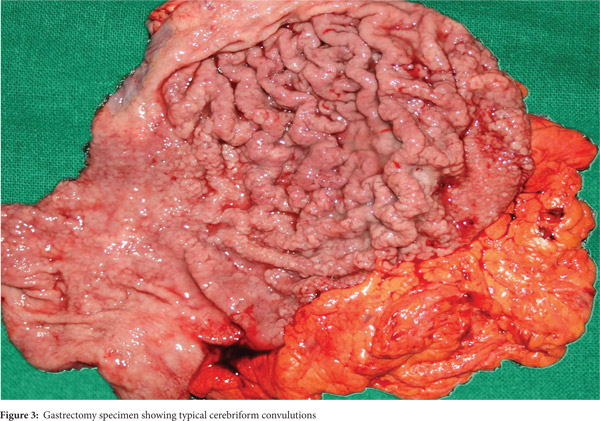
 Subsequently, multiple snare biopsies of the stomach were taken. These showed evidence of chronic gastritis with foveolar hyperplasia, dysplastic glands, reversal of pit-gland ratio and areas of intestinal metaplasia with foci of well differentiated adenocarcinoma (Figure 2). A contrast enhanced CT scan of the abdomen confirmed thickened mucosal folds involving the fundus and body of the stomach with antral sparing. It also revealed symmetric enhancement of hypertrophic gastric mucosa (Figure 1b). CT did not show any abnormal enhancing mass lesion. He was planned for, and underwent a total gastrectomy with an uneventful postoperative period. The mucosal surface of the resected stomach exhibited typical cerebriform convolutions (Figure 3) and histopathology confirmed features of Menetrier’s disease with foci of well differentiated adenocarcinoma.
Subsequently, multiple snare biopsies of the stomach were taken. These showed evidence of chronic gastritis with foveolar hyperplasia, dysplastic glands, reversal of pit-gland ratio and areas of intestinal metaplasia with foci of well differentiated adenocarcinoma (Figure 2). A contrast enhanced CT scan of the abdomen confirmed thickened mucosal folds involving the fundus and body of the stomach with antral sparing. It also revealed symmetric enhancement of hypertrophic gastric mucosa (Figure 1b). CT did not show any abnormal enhancing mass lesion. He was planned for, and underwent a total gastrectomy with an uneventful postoperative period. The mucosal surface of the resected stomach exhibited typical cerebriform convolutions (Figure 3) and histopathology confirmed features of Menetrier’s disease with foci of well differentiated adenocarcinoma.
 Discussion
Menetrier’s Disease is a hypertrophic gastropathy believed to be caused by an overexpression of tumour growth factor a (TGF-a), a ligand for the tyrosine kinase epidermal growth factor receptor, resulting in selective expansion of surface mucous cells and hypersecretion of mucus and hypochlorhydria which also predisposes to a malignancy.[1] It affects mostly adults, but can also occur in children. The average age at diagnosis in adults is 55 years, with male preponderance. Menetrier’s disease should be distinguished from other hypertrophic gastropathies such as the Zollinger–Ellison syndrome, lymphocytic gastritis, and diffuse-type adenocarcinoma (linitis plastica), all of which exhibit generalized rugal hypertrophy. The hyperplastic changes seen in Menetrier’s disease typically involve the body and/or the fundus of the stomach but spare the antrum, as was the finding in our patient.
The most common symptoms include epigastric pain with fullness, nausea and vomiting, generalized peripheral edema and hypoalbuminemia due to leakage of protein selectively across the gastric epithelial lining. The disease tends to be progressive in adults.[1] An esophagogastroduodenoscopy, one of the initial investigations, will show markedly increased thickening of gastric rugal folds, resembling cerebral convolutions, often with surface erosions accompanied by copious amounts of thick mucus that may form bridges across the gastric lumen obscuring visualization.[3] Deep snare biopsies rather than punch biopsies allow accurate assessment of mucosal architecture and of the pit to gland ratio. Foveolar hyperplasia, dilated and tortuous glands, significant parietal cell loss, smooth muscle hyperplasia and reversal of pit-gland ratio are features of Menetrier’s disease.[3] All of these features were seen in our patient as well.
A transabdominal ultrasonogram depicts gastric wall thickening and stratification and is a useful non-invasive modalityforthedifferentialdiagnosisofgiantgastricfolds.[4] Multidetector CT (MDCT) is a reliable non-invasive diagnostic modality in the differentiation of diseases with giant gastric folds. It is often difficult at endoscopy with conventional mucosal biopsies to differentiate Menetrier’s disease from other common diseases characterized by hypertrophic rugal folds.[5] MDCT with multiplanar reformation (MPR) images enables accurate assessment of the thickness, enhancement pattern, wall stratification and horizontal extension and depth of tumour invasion. It displays information distant from the stomach and aids in performing additional biopsies. Menetrier’s disease displays thick mucosal enhancement and absent submucosal enhancement in the arterial phase with preservation of wall stratification, while linitis plastica and lymphomas exhibit submucosal enhancement along with loss of gastric wall stratification.[5] The CT scan of our patient depicted all these typical features.
Menetrier’s disease is a premalignant condition and though almost 200 cases of the disease have been reported in the literature, only few cases have been associated with gastric adenocarcinoma. Factors such as hypochlorhydria and antral atrophy may predispose to gastric cancer as a consequence of bacterial overgrowth and a concomitant increment in carcinogenic substances such as nitrosamines. Owing to its premalignant potential, an endoscopy surveillance programme would be a useful recommendation for patients with Menetrier’s disease, though sampling errors may limit its efficacy. Recent improvements in endoscopic techniques and increased understanding and recognition of early gastric cancer (EGC) are important, but the usefulness of these techniques in Menetrier’s disease remains unknown.[6]
Treatment options for Menetrier’s disease includes antacids, proton pump inhibitors, somatostatin analogues, steroids and more recently, a monoclonal antibody to EGFR, Cetuximab has been found in preliminary reports to have a good clinical and biochemical response. Surgery is indicated for persistent and refractory symptoms like generalized edema, abdomen pain and nausea or as in our case, presence of malignancy. Total gastrectomy is preferred over partial gastrectomy owing to the risk of malignancy and better postoperative outcomes.[1,7]
References
Discussion
Menetrier’s Disease is a hypertrophic gastropathy believed to be caused by an overexpression of tumour growth factor a (TGF-a), a ligand for the tyrosine kinase epidermal growth factor receptor, resulting in selective expansion of surface mucous cells and hypersecretion of mucus and hypochlorhydria which also predisposes to a malignancy.[1] It affects mostly adults, but can also occur in children. The average age at diagnosis in adults is 55 years, with male preponderance. Menetrier’s disease should be distinguished from other hypertrophic gastropathies such as the Zollinger–Ellison syndrome, lymphocytic gastritis, and diffuse-type adenocarcinoma (linitis plastica), all of which exhibit generalized rugal hypertrophy. The hyperplastic changes seen in Menetrier’s disease typically involve the body and/or the fundus of the stomach but spare the antrum, as was the finding in our patient.
The most common symptoms include epigastric pain with fullness, nausea and vomiting, generalized peripheral edema and hypoalbuminemia due to leakage of protein selectively across the gastric epithelial lining. The disease tends to be progressive in adults.[1] An esophagogastroduodenoscopy, one of the initial investigations, will show markedly increased thickening of gastric rugal folds, resembling cerebral convolutions, often with surface erosions accompanied by copious amounts of thick mucus that may form bridges across the gastric lumen obscuring visualization.[3] Deep snare biopsies rather than punch biopsies allow accurate assessment of mucosal architecture and of the pit to gland ratio. Foveolar hyperplasia, dilated and tortuous glands, significant parietal cell loss, smooth muscle hyperplasia and reversal of pit-gland ratio are features of Menetrier’s disease.[3] All of these features were seen in our patient as well.
A transabdominal ultrasonogram depicts gastric wall thickening and stratification and is a useful non-invasive modalityforthedifferentialdiagnosisofgiantgastricfolds.[4] Multidetector CT (MDCT) is a reliable non-invasive diagnostic modality in the differentiation of diseases with giant gastric folds. It is often difficult at endoscopy with conventional mucosal biopsies to differentiate Menetrier’s disease from other common diseases characterized by hypertrophic rugal folds.[5] MDCT with multiplanar reformation (MPR) images enables accurate assessment of the thickness, enhancement pattern, wall stratification and horizontal extension and depth of tumour invasion. It displays information distant from the stomach and aids in performing additional biopsies. Menetrier’s disease displays thick mucosal enhancement and absent submucosal enhancement in the arterial phase with preservation of wall stratification, while linitis plastica and lymphomas exhibit submucosal enhancement along with loss of gastric wall stratification.[5] The CT scan of our patient depicted all these typical features.
Menetrier’s disease is a premalignant condition and though almost 200 cases of the disease have been reported in the literature, only few cases have been associated with gastric adenocarcinoma. Factors such as hypochlorhydria and antral atrophy may predispose to gastric cancer as a consequence of bacterial overgrowth and a concomitant increment in carcinogenic substances such as nitrosamines. Owing to its premalignant potential, an endoscopy surveillance programme would be a useful recommendation for patients with Menetrier’s disease, though sampling errors may limit its efficacy. Recent improvements in endoscopic techniques and increased understanding and recognition of early gastric cancer (EGC) are important, but the usefulness of these techniques in Menetrier’s disease remains unknown.[6]
Treatment options for Menetrier’s disease includes antacids, proton pump inhibitors, somatostatin analogues, steroids and more recently, a monoclonal antibody to EGFR, Cetuximab has been found in preliminary reports to have a good clinical and biochemical response. Surgery is indicated for persistent and refractory symptoms like generalized edema, abdomen pain and nausea or as in our case, presence of malignancy. Total gastrectomy is preferred over partial gastrectomy owing to the risk of malignancy and better postoperative outcomes.[1,7]
References
- Coffey RJ Jr, Tanksley J. Pierre Ménétrier and his disease. Trans Am Clin Climatol Assoc. 2012;123:126-33; discussion 133-4.
- Chung M, Pittenger J, et al. Atypical Clinical and Diagnostic Features in Ménétrier’s Disease in a child. Case Rep Gastrointest Med, 2011: 480610
- Rich A, Toro TZ, Tanksley J, et al. Distinguishing Menetrier’s disease from its mimics. Gut. 2010;59:1617–24.
- Okanobu H, Hata J, Haruma K, et al. Giant gastric folds: differential diagnosis at US. Radiology. 2003, 226:686–690.
- Chen CY, Jaw TS, Wu DC, et al. MDCT of Giant Gastric Folds: differential diagnosis. AJR. 2010; 195(5): 1124-1130
- Remes-Troche, J. M. et al. Early gastric cancer in Menetrier’sdisease.BMJCaseRep2009:ber07.2008.0453.
- Toubia N, Schubert ML. Menetrier’s disease. Curr Treat Options Gastroenterol. 2008;11:103–108.
|