Joshua Franklyn1, Rohin Mittal1, Tunny Sebastian2, Benjamin Perakath1
1Department of Surgery Unit 2 (Colorectal Surgery)
Christian Medical College, Vellore
2Department of Biostatistics,
Christian Medical College,
Vellore
Corresponding Author:
Rohin Mittal
Associate Professor
Department of Surgery Unit 2
(Colorectal Surgery)
Christian Medical College,
Vellore
Tamilnadu 632004
India
Email :rohinmittal@gmail.com
Abstract
Background: There is sparse Indian data on right colon cancer. Available literature suggests that it affects the young and survival is poor.
Aim: This article reviews demographics and outcomes of surgically treated right sided colon cancer over a nine year period in a single colorectal unit in a tertiary care teaching hospital.
Methods: A retrospective review of all patients undergoing right hemicolectomy for adenocarcinoma of the colon between January 2004 and December 2012 was undertaken. Data was collected from hospital records and telephonic interview when possible.
Results: Two hundred and thirteen patients were studied. Mean age was 49 years with 57.1% being 50 years or younger. Stage 1 disease was seen in 9.9%, stage 2 in 35.2%, stage 3 in 42.3%, and stage 4 in 12.7%. Follow up was available for 81.6% with a mean follow up of 35.6 months. Five year disease free survival (DFS) and overall survival (OS) was 81% and 74%. Presence of lymphovascular invasion and age >50 years were predictors of poor survival. Poor prognostic features on histopathology were not different between the young and the old. The 5 year DFS was similar in both, but the 5 year OS was better for the young (90% vs. 73%, p=0.029).
Conclusions: Patients with right colon cancer are younger in India. They have similar histopathology when compared to the older population. Operable right colon cancer has an excellent prognosis. Five year DFS is similar in the young and the old, but OS is lower in the older population.
|
48uep6bbphidcol2|ID 48uep6bbphidvals|1700 48uep6bbph|2000F98CTab_Articles|Fulltext Colorectal cancer is the third most common cancer in men and the second most common cancer in women worldwide[1]. The incidence of colon cancer in India is much lower; it is the ninth most common cancer among men and women[2]. In India, the incidence of colonic malignancies ranges from 0.7 - 3.7/100,000 among men and 0.4 - 3/100,000 among women[3]. Recent reports suggest that while the incidence of colon cancer is rising in Asia, India is an exception where the incidence still remains low4. However, the low incidence rates may be susceptible to bias as there may be a high degree of underreporting[4]. There have also been recent reports suggesting that India has a higher proportion than expected of under-40 patients with colorectal cancer[5].
The demographics of right colon cancer in India are different from the West, with most patients being from a rural background and a lower socio economic strata. Variations in lifestyle and dietary habits play an important role in the difference in demographics[4].
Right colon cancer has good long term survival, with the overall survival being as high as 64%.[6] However, the reported 5-year survival in India has been poor, even with early colon cancers, when compared to other Asian countries and the West[7,8].
This paper analyses the demographics and outcomes of surgically treated right colon cancer over a 9-year period at a colorectal unit in a tertiary care referral teaching center in South India.
Methods
This was a retrospective review of all patients undergoing right hemicolectomy for primary adenocarcinoma of the colon in a single colorectal unit of a tertiary care teaching hospital between January 2004 and December 2012.
All patients undergoing right hemicolectomy (limited, standard or extended) for primary adenocarcinoma of the colon were included in the study. Patients were staged based on the TNM classification as defined by the AJCC 7th edition[9].
Data on demographics, staging, type of operation, histopathology and outcome was collected from electronic and paper hospital records. Follow up was obtained from electronic hospital records and by telephonic interview.
The data was analyzed using SPSS for windows, version 16.0. Chicago, SPSS Inc. Statistical significance for individual variables was analyzed using the chi-square and Fisher’s exact test for categorical variables and the t-test for continuous variables. Survival analysis was done using Kaplan Meier curves and compared using the Log Rank (Mantel-Cox) test.
Results
Two hundred and thirteen patients underwent right hemicolectomy for primary adenocarcinoma of the colon during the study period. 151 (70.9%) were male and 62 (29.1%) were female, with a male-to-female ratio of 2.4:1.
The mean age at presentation was 49 years (Range 15-80, SD 12.2) and 57.1 % were 50 years or younger. The age distribution is shown in Figure 1. The mean age of presentation was 49.4 years (SD 12) among males and 47.9 years (SD 12.7) among females.
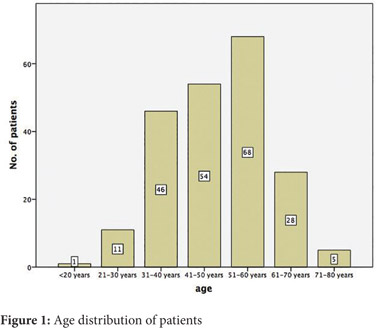 The primary was in the caecum in 47 patients (22.1%), the ascending colon in 99(46.5%), the hepatic flexure in 41(19.2%) and the transverse colon in 26 (12.2%). A standard right hemicolectomy was performed in 163 patients (76.5%), 4 (1.9%) underwent a limited right while 46 (21.6%) had an extended right hemicolectomy. 205 (96.2%) patients were anastomosed.
The primary was in the caecum in 47 patients (22.1%), the ascending colon in 99(46.5%), the hepatic flexure in 41(19.2%) and the transverse colon in 26 (12.2%). A standard right hemicolectomy was performed in 163 patients (76.5%), 4 (1.9%) underwent a limited right while 46 (21.6%) had an extended right hemicolectomy. 205 (96.2%) patients were anastomosed.
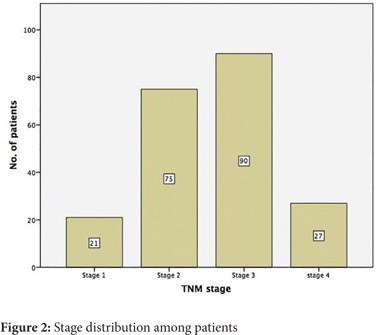
On staging, 2 patients (0.9%) were T1, 23 (10.8%) were T2, 138 (64.8%) were T3 and 50(23.5%) were T4. Node positive disease was present in 112 (52.6%) patients and metastatic disease in 27 (12.7%). Final TNM staging revealed 21 (9.9%) patients to be stage 1, 75 (35.2%) as stage 2, 90 (42.3%) as stage 3, and 27(12.7%) as stage 4 (Figure 2). 161 (75.6%) patients had more than 12 nodes in the specimen.
 19 patients had an anastomotic leak and the anastomotic leak rate was 9.3%. The 30-day mortality was 2.3% (5/213).
19 patients had an anastomotic leak and the anastomotic leak rate was 9.3%. The 30-day mortality was 2.3% (5/213).
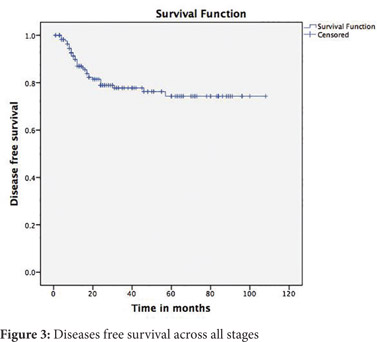
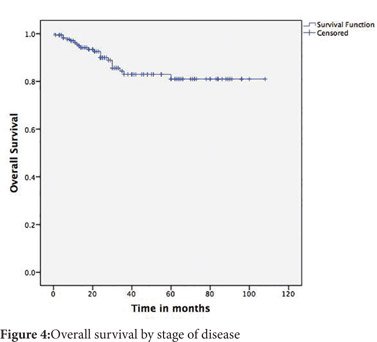
Follow up was available for 174(81.6%) patients. The mean follow up was 35.6 months (SD 27 months). The 5-year disease free survival (DFS) across all stages was 74% and the overall survival (OS) was 81% (Figure 3 and 4). The 5-year DFS and OS were 94% and 91% for stage 2, 65% and 77% for stage 3 and nil for stage 4 (Figure 5 and 6). There was no mortality or recurrence in the few patients with stage 1 disease.
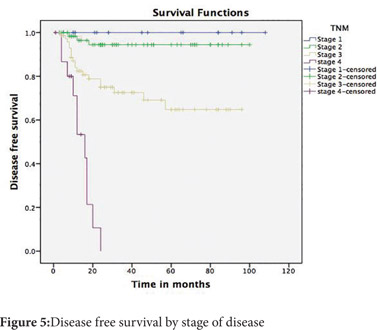
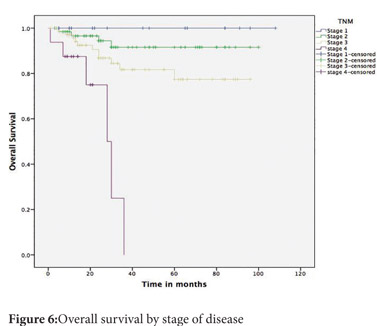
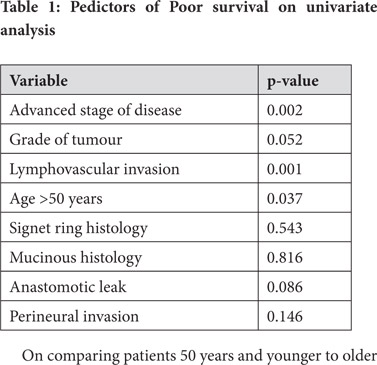
The stage of the disease, the grade of the tumour, the presence of lymphovascular invasion and age more than 50 years were predictors of poor survival on univariate analysis (Table 1). On multivariate analysis, the presence of lymphovascular invasion and age more than 50 years were found to be significant predictors of poor survival.
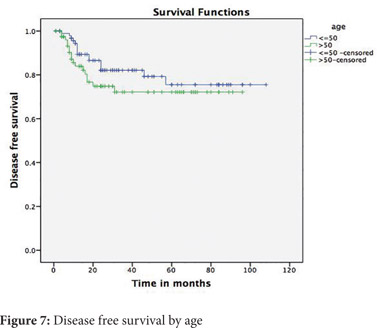
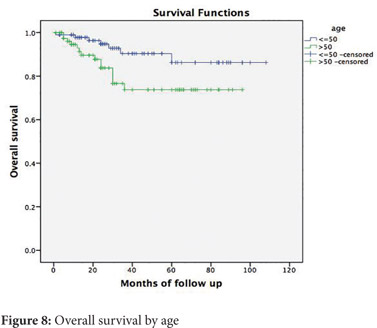
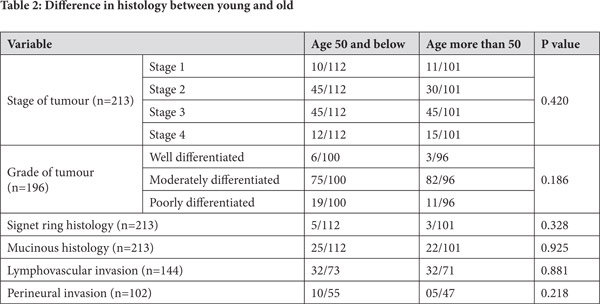
 On comparing patients 50 years and younger to older patients, no significant differences were observed in the stage of tumour, the grade of tumour, signet ring histology, mucinous histology or lymphovascular and perineural invasion (Table 2). The 5-year disease free survival for younger patients was 82% and for older patients was 72% (p=0.226) (Figure 7). The 5-year overall survival for younger patients was 90% and for older patients was 73 % (p=0.029) (Figure 8).
On comparing patients 50 years and younger to older patients, no significant differences were observed in the stage of tumour, the grade of tumour, signet ring histology, mucinous histology or lymphovascular and perineural invasion (Table 2). The 5-year disease free survival for younger patients was 82% and for older patients was 72% (p=0.226) (Figure 7). The 5-year overall survival for younger patients was 90% and for older patients was 73 % (p=0.029) (Figure 8).
Discussion
Colon cancer is classically described as a disease of the elderly. The median age of presentation of right colon cancer as described by Benedix et. al. was 71 years[10]. However, it has recently been observed that colorectal cancer is increasingly being diagnosed in a younger population.[5,11,12] The fact that 57.1% of patients with operable right colon cancer were aged 50 years or younger in our series is in keeping with this observed trend. This is in contrast to Western literature where more than 90% of colorectal cancer occurs in people aged over 50[13]. The median age in our series is about two decades younger than in the West. The reasons for this early presentation of sporadic colon cancer are poorly understood, and further studies, including genetic analysis may be necessary to understand this phenomenon.
What is interesting to note is that colon cancer in the young as well as the older patients seem to have similar histopathological features. The stage of the disease, the grade of the tumour, the presence of lymphovascular and perineural invasion as well as signet ring and mucinous histopathology are no different in the two groups. This is in conflict with some of the other reports which say that younger patients have higher stages as well as grades of tumours[13].
Opinions on the survival of young patients with colon cancer have been divided. The SEER data base concluded in 2004 that the 5-year survival was better for young patients with colorectal cancer[14]. This has also been corroborated by Mcmillan et. al[15]. In other reports, young adults with colon cancer were at an increased risk of recurrence and cancer related deaths even after 5 years.[16,17] In our study, 5-year disease free survival was not different in the two groups. The young patients, however, had a statistically significant higher overall survival. This is probably a reflection of a higher mortality in the elderly secondary to non-cancer causes.
The stage distribution of colon cancer seen in India is also different from the West. Early stage tumours (T1 and T2) are more common in the west, but are uncommon in our population. One report from the west reveals 38% localized disease and 37% regional disease[18]. In contrast, our study reveals only 9.9% stage 1 tumours, with 77.5% stage 2 and 3 tumours. The reasons for presentation at an advanced stage may include lack of awareness of the symptoms of colon cancer, inadequate access to health care or the lack of a screening program. Younger patients may also present with advanced disease as symptoms may not be attributed to malignancy due to a low index of suspicion[19]. Also, patients may be treated symptomatically without thorough investigations, thus delaying the diagnosis.
The 5-year overall survival of colon cancer has been reported to be about 65% in Western literature.[18,20] However, survival data about operable right colon cancer from India is very scanty. The reported 5-year survivals are poor. The overall survival from the Bombay cancer registry was only 30%.[4] What was even more alarming is that the 5-year survival of early colon carcinoma was only 60%.[4] The figures from Bhopal were also low, with an overall 5-year survival (all stages) of 5%.[4] This is in contrast to our report, where we report excellent outcomes in patients with operable right colon cancer. The 5-year overall survival of operable right colon cancer, stage for stage, in our series is comparable to Western data. This success rate may be attributed to proper pre-operative staging, standardized operative techniques and the use of adjuvant chemotherapy in our patients.
Presently, colon cancer is not a major health problem in India which warrants surveillance. However, with increased urbanization and lifestyle changes, it is likely to gain importance.[21] In view of the young age at presentation, if screening for a high risk group is considered in India, we predict that the average age of starting screening will be less than that in the Western population.
Limitations of this study include its retrospective nature and short duration of follow up. In addition, as this data is from a single tertiary care centre, there may be considerable referral bias. As we have included only operable cases, data on stage 4 disease may be incomplete. While we have specifically studied only right colon cancer, many of the studies used to compare results have looked at colon cancers as a whole or colorectal cancers in entirety. The demographics and prognosis of right and left sided colon cancers are not the same and data on right colon cancer alone is sparse.
Conclusion
The demographics of right colon cancer in India are different from the West. Patients present at a younger age. However, the stage of presentation and histopathology in such patients do not differ from older patients. When treated with curative intent, right colon cancer has an excellent prognosis. In contrast to previous reports from India, we have shown that, stage for stage, survival is comparable to the West. We have also shown that younger patients have better overall survival, but comparable disease free survival to older patients.
References
- International agency for research on cancer. GLOBOCAN 2012: Estimated cancer incidence, Mortality and prevalence worldwide in 2012 [Internet]. globocan.iarc.fr. Available from: http:// globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- Sirohi B, Bhatia V, Julka P, Lele V, Chaturvedi A, Kaur T, et al. Indian Council of Medical Research consensus document for the management of colorectal cancer. Indian J Med Paediatr Oncol. 2014;35:192.
- Mohandas KM, Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol Off J Indian Soc Gastroenterol. 1999 Sep;18:118–21.
- Pathy S, Lambert R, Sauvaget C, Sankaranarayanan R. The incidence and survival rates of colorectal cancer in India remain low compared with rising rates in East Asia. Dis Colon Rectum. 2012 Aug;55:900–6.
- Nath J, Wigley C, Keighley MRB, Perakath B. Rectal cancer in young adults: a series of 102 patients at a tertiary care centre in India. Colorectal Dis. 2009 1;11:475–9.
- Cancer Facts & Figures - 2013 - acspc-036845. pdf [Internet]. [cited 2014 Oct 30]. Available from: http://www.cancer.org/acs/groups/content/@ epidemiologysurveilance/documents/document/ acspc-036845.pdf.
- Moghimi-Dehkordi B, Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol. 2012; 4:71–5.
- Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X-S, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). The Lancet [Internet]. 2014 Nov [cited 2014 Dec 10]; Available from: http://www.thelancet.com/journals/ lancet/article/PIIS0140-6736(14)62038-9/abstract.
- Edge SB, Byrd, D.R., Compton, C.C. AJCC Cancer Staging Manual 7th ed. 2010.
- Benedix F, Schmidt U, Mroczkowski P, Gastinger I, Lippert H, Kube R, et al. Colon carcinoma-- classification into right and left sided cancer or according to colonic subsite?--Analysis of 29,568 patients. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2011;37:134–9.
- Shaikh AJ, Raza S, Shaikh AA, Idress R, Kumar S, Rasheed YA, et al. Demographics, pathologic patterns and long-term survival in operable colon cancers: local experience in Pakistan. Asian Pac J Cancer Prev APJCP. 2009;10:361–4.
- Young JP, Win AK, Rosty C, Flight I, Roder D, Young GP, et al. Rising incidence of early-onset colorectal cancer in Australia over two decades: Report and review. J Gastroenterol Hepatol. 2015;30:6–13.
- O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Do young colon cancer patients have worse outcomes? World J Surg. 2004 ;28:558–62.
- Li Q, Cai G, Li D, Wang Y, Zhuo C, Cai S. Better Long-Term Survival in Young Patients with Non-Metastatic Colorectal Cancer after Surgery, an Analysis of 69,835 Patients in SEER Database. PLoS ONE. 2014;9:e93756.
- McMillan DC, McArdle CS. The impact of young age on cancer-specific and non-cancer-related survival after surgery for colorectal cancer: 10-year follow-up. Br J Cancer. 2009;101:557–60.
- Leff DR, Chen A, Roberts D, Grant K, Western C, Windsor ACJ, et al. Colorectal cancer in the young patient. Am Surg. 2007;73:42–7.
- Fu J-F, Huang Y-Q, Yang J, Yi C-H, Chen H-L, Zheng S. Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol WJG. 2013;19:8078–84.
- Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17.
- Ben-Ishay O, Brauner E, Peled Z, Othman A, Person B, Kluger Y. Diagnosis of colon cancer differs in younger versus older patients despite similar complaints. Isr Med Assoc J IMAJ. 2013;15:284–7.
- Browse the SEER Cancer Statistics Review 1975-2011 [Internet]. 2015 [cited 2015 Jan 11]. Available from: http://seer.cancer.gov/csr/1975_2011/browse_csr. php?sectionSEL=6&pageSEL=sect_06_table.13.html
- Sung JJY, Lau JYW, Young GP, Sano Y, Chiu HM, Byeon JS, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008:1166–76.
|